Tertiary phosphines constitute an important class of compounds in organic synthesis. Apart from being essential ligands [1] for metal ligation, they are versatile reagents for various organic reactions [2], such as the Wittig, Mitsunobu, Appel, and Staudinger reactions, etc. Among the various procedures available for phosphine synthesis [3], the reduction of tertiary phosphine oxides is an attractive and viable route that is also a method for recycling phosphine resources [4].
To date, many procedures for the reduction of tertiary phosphine oxides have been reported. Systems such as aluminum hydrides [5], silanes [6] or hydrosiloxanes [7] with transition metal catalysts and low-valent transition metals [8] have been used in the direct reduction of tertiary phosphine oxides. On the other hand, the indirect reduction of tertiary phosphine oxides to tertiary phosphines via the corresponding tertiary phosphine dichlorides has also been described, in which the intermediate tertiary phosphine dichlorides is reduced with different metal agents, or by electroreduction [9]. Though those methods mentioned above provide the desired product, different kinds of metal agents have to be used.
Metal-free reductions, as highly promising candidates for green and sustainable transformations, are important alternatives to conventional metal-mediated reductions. Thus far, several metalfree reductions of tertiary phosphine oxides to tertiary phosphines have been reported. Silanes have also been used and give the expected phosphines with good yields, but unfortunately several silanes have demonstrated to generate dangerous, pyrophoric and toxic SiH4 gas [10]. Non-metallic organic reagents or hydrogenation of dichloride derivatives are also not recommended because of their harsh conditions [11]. Reduction with thiols generates phosphine sulfide as a by-product, but the strong and repulsive odor of thiols also make the operation difficult [12]. Based on the above-mentioned considerations, developing new methods for the reduction of tertiary phosphine oxides to tertiary phosphines under metal-free conditions is of promising interest.
Hantzsch esters (HEHs) are easily available as bio-inspired hydride donors. They have been widely used in the hydrogenation of C=C, C=N, and C=O bonds in the presence of organocatalysts, or metal catalysts [13]. Initially, the knowledge that HEHs can deliver the hydride or hydrogen-atom attracted our attention [14]. We surmised that HEHs may be used as efficient reductants for the reduction of P=O bonds. In this paper, we report on the one-pot reduction of tertiary phosphine oxides to tertiary phosphines under metal-free conditions.
All reagents and solvents were obtained from commercial sources and were used without further purification. Column chromatography was performed with silica gel (200-300 mesh). Thin layer chromatography was carried out using Merck silica gel GF254 plates. The 31P NMR, 1H NMR and 13C-NMR spectra were measured on Bruker 400 M spectrometers with 1H NMR and 13C-NMR recorded in CDCl3 using tetramethylsilane (TMS) as the internal standard. For 31P NMR, 85% H3PO4 was used as external standard.
General procedure for the reduction of tertiary phosphine oxides: To a solution of tertiary phosphine oxides 2 (0.4 mmol) in CH2Cl2 (2 mL) was added oxalyl chloride (0.4 mmol) dropwise at room temperature under an argon atmosphere. After 30 min, HEH (1 mmol) was added to the mixture in one portion as well as TEA (3 mmol). The mixture was stirred for another 2 h at 40 ℃, and then diluted with H2O (10 mL). The resulting mixture was extracted with CH2Cl2 repeatedly. The extracts were dried (anhydrous Na2SO4) and evaporated. The crude product was purified by column chromatography on silica gel using ethyl acetate/petroleum ether to afford the pure product.
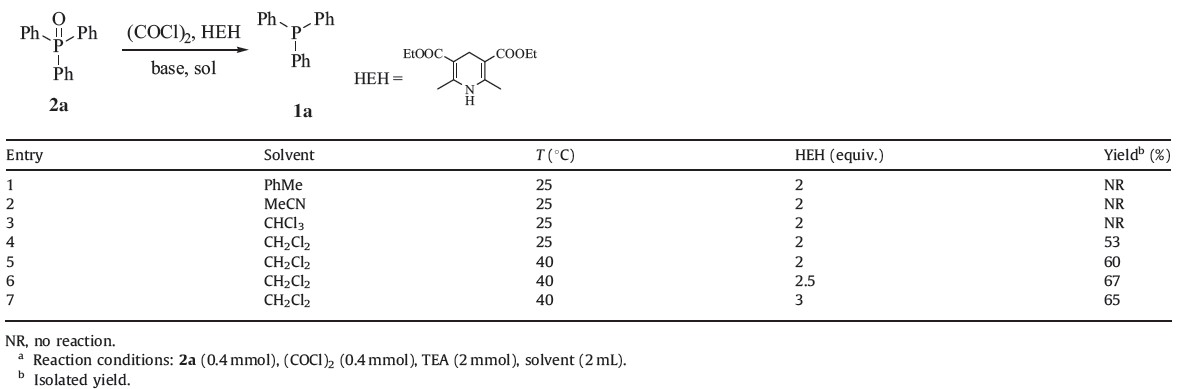
Activation of tertiary phosphine oxides was effected by chlorination with oxalyl chloride [15]. During optimization studies, triphenylphosphine oxide 2a was chosen as the model substrate and, initially, reaction solvents were screened. To a solution of 1 equiv. of 2a dissolved in different solvents (2 mL) was added 1 equiv. of oxalyl chloride dropwise at room temperature under an argon atmosphere (Table 1). After 30 min, various equiv. of HEH were added to the mixture in one portion as well as 5 equiv. of triethylamine (TEA). The mixture was stirred for 2 h at ambient temperature to afford 1a.
The results in Table 1 indicated that solvents exhibited decisive effects on the reaction (Table 1, entries 1-4). No product was detected in toluene, acetonitrile and chloroform (Table 1, entries 1-3) even though the reaction time was prolonged to 12 h. Dichloromethane (Table 1, entry 4), however, gave the product in moderate yield (53%). When the temperature was increased from room temperature to 40 ℃, the yield improved to 60% (Table 1, entry 5). The yield was further increased to 67% upon the addition of 2.5 equivalents of HEH (Table 1, entry 6). Adding more HEH failed to improve the product yield (Table 1, entry 7).
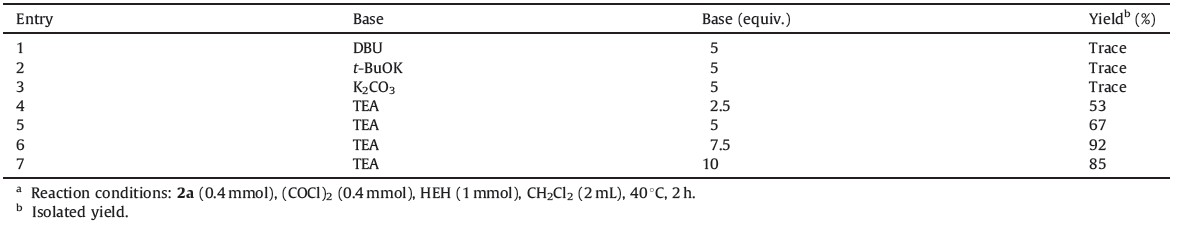
Subsequently, different bases were examined (Table 2, entries 1-4). The use of DBU (1, 8-diazabicyclo[5.4.0]undec-7-ene), t-BuOK and K2CO3 did not give any product. However, TEA (7.5 equivalents) gave the best yield of 92% (Table 2, entry 6) compared with others (Table 2, entries 4, 5, 7).
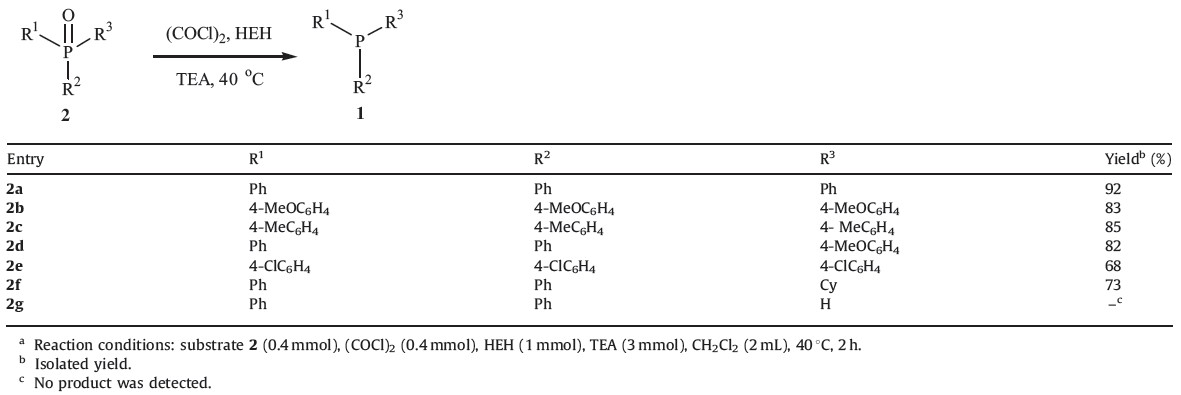
With the optimized conditions determined, we then applied this metal-free methodology to the reduction of other phosphine oxides to the corresponding phosphines (Table 3). Triarylphosphine oxides with electron-donating groups 2b, 2c and the unsymmetrical oxide 2d proceeded smoothly to give the corresponding phosphines 1b-1d in 82-85% yields. Tris(4- chlorophenyl)phosphine oxide 2e was reduced less effectively to give phosphine 1e in 68% yield. Reduction of cyclohexyldiphenylphosphine oxide 2f gave the corresponding phosphine in 73% yield. Application of this metal-free reduction method to secondary phosphine oxide 2g gave no product. The NMR spectra and data of compounds 1a-f are available in Supporting information.
| Table 1 One-pot reduction of 2a to 1a by using (COCl)2/HEH system.a |
| Table 2 Effect of base on the reduction of 2a to 1a. |
| Table 3 Reduction of tertiary phosphine oxides.a |
In summary, we have developed a new metal-free method for the reduction of tertiary phosphine oxides to tertiary phosphines. The (COCl)2/HEH method has proven to be an effective system for the reduction of triarylphosphine oxides and alkyldiarylphosphine oxides to the corresponding phosphines, and constitutes an alternative to the reported reduction of phosphine oxides or recycling of phosphine resources.
We thank the Natural Science Foundation of China (Nos. 21021001, 21072134 and J1103315/J0104) for financial support and the Analytical & Testing Center of Sichuan University for NMR measurements.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2013.09.007.
| [1] | (a) M. Berthod, G. Mignani, G. Woodward, M. Lemaire, Modified binap: the how and the why, Chem. Rev. 105 (2005) 1801-1836; (b) M.L. Ma, Z.H. Peng, Y. Guo, et al., Alkoxy substituted MeO-BIPHEP-type diphosphines ligands for asymmetric hydrogenation of aryl ketones, Chin. Chem. Lett. 21 (2010) 576-579. |
| [2] | (a) D.L. Wang, Z. Dong, J. Xu, D. Li, An efficient synthesis of 2-(guaiazulen-1-yl)furan derivatives via intramolecular Wittig reactions, Chin. Chem. Lett. 24 (2013) 622-624; (b) B.E. Maryanoff, A.B. Reitz, The Wittig Olefination reaction and modifications involving phosphoryl-stabilized carbanions. stereochemistry, mechanism, and selected synthetic aspects, Chem. Rev. 89 (1988) 863-927. |
| [3] | (a) J.J. Feng, X.F. Chen, M. Shi, W.L. Duan, Palladium-catalyzed asymmetric addition of diarylphosphines to enones toward the synthesis of chiral phosphines, J. Am. Chem. Soc. 132 (2010) 5562-5563; (b) X. Wang, Z. Han, Z. Wang, K. Ding, Catalytic asymmetric synthesis of aromatic spiroketals by spinphox/iridium(Ⅰ)-catalyzed hydrogenation and spiroketalization of α,α'-bis(2-hydroxyarylidene) ketones, Angew. Chem. Int. Ed. 51 (2012) 936-940. |
| [4] | H. Tanaka, T. Yano, K. Kobayashi, et al., TMSCl-promoted electroreduction of triphenylphosphine oxide to triphenylphosphine, Synlett 24 (2011) 582-584. |
| [5] | T. Imamoto, S. Kikuchi, T. Miura, Y. Wada, Stereospecific reduction of phosphine oxides to phosphines by the use of a methylation reagent and lithium aluminum hydride, Org. Lett. 3 (2001) 87-90. |
| [6] | (a) H.A. Van Kalkeren, S.H.A.M. Leenders, C.R.A. Hommersom, F.P.J.T. Rutjes, F.L. Van Delft, In situ phosphine oxide reduction: a catalytic Appel reaction, Chem. Eur. J. 17 (2011) 11290-11295; (b) Y. Segall, I. Granoth, A. Kalir, Preparation of a dibenzo[b,e]phosphorin, selective phosphine oxide reduction by trichlorosilane, J. Chem. Soc. Chem. Comm. (1974) 501-502; (c) W.R. Davis, M.D. Gordon, Reduction of phosphine oxides, US 4131624, 1978. (d) T. Coumbe, N.J. Lawrence, F. Muhammad, Titanium (IV) catalysis in the reduction of phosphine oxides, Tetrahedron Lett. 35 (1994) 625-628. |
| [7] | (a) M. Berthod, A. Favre-Réguillon, J. Mohamad, et al., A catalytic method for the reduction of secondary and tertiary phosphine oxides, Synlett 10 (2007) 1545-1548; (b) C. Petit, A. Favre-Réguillon, G. Mignani, M. Lemaire, A straightforward synthesis of unsymmetrical secondary phosphine boranes, Green Chem. 12 (2010) 326-330; (c) C. Petit, A. Favre-Réguillon, B. Albela, et al., Mechanistic insight into the reduction of tertiary phosphine oxides by Ti(OiPr)4/TMDS, Organometallics 28 (2009) 6379-6382; (d) Y. Li, S. Das, S. Zhou, K. Junge, M. Beller, General and selective coppercatalyzed reduction of tertiary and secondary phosphine oxides: convenient synthesis of phosphines, J. Am. Chem. Soc. 134 (2012) 9727-9732; (e) L. Pehlivan, E. Metay, D. Delbrayelle, G. Mignani, M. Lemaire, Reduction of phosphine oxides to phosphines with the InBr3/TMDS system, Tetrahedron 68 (2012) 3151-3155. |
| [8] | M. Zabiocka, B. Delest, A. Igau, A. Skowronska, J.P. Majoral, [Cp2ZrHCl]n a useful reducing agent in phosphorus chemistry, Tetrahedron Lett. 38 (1997) 5997-6000. |
| [9] | K.V. Rajendran, D.G. Gilheany, Simple unprecedented conversion of phosphine oxides and sulfides to phosphine boranes using sodium borohydride, Chem. Commun. 48 (2012) 817-819. |
| [10] | S.C. Berk, S.L. Buchwald, An air-stable catalyst system for the conversion of esters to alcohols, J. Org. Chem. 58 (1993) 3221. |
| [11] | V.T. Dockner, Reduktion und hydrierung mit dem system kohlenwasserstoff/ kohlenstoff, Angew. Chem. Int. Ed. 100 (1988) 699-702. |
| [12] | M. Masaki, K. Fukui, Reaction of tertiary phosphine dichlorides with thiols in the presence of triethylamine, a convenient method for the reduction of phosphine oxides to phosphines, Chem. Lett. (1977) 151-152. |
| [13] | M. Zhang, H.W. Yang, Y. Zhang, et al., Direct reductive amination of aromatic aldehydes catalyzed by gold(Ⅰ) complex under transfer hydrogenation conditions, Chem. Commun. 47 (2011) 6605-6607. |
| [14] | X.Q. Zhu, H.R. Li, Q. Li, et al., Determination of the C4-H bond dissociation energies of NADH models and their radical cations in acetonitrile, Chem. Eur. J. 9 (2003) 871-880. |
| [15] | O. Mó, M. Yáñez, M. Eckert-Maksić, et al., Periodic trends in bond dissociation energies, J. Phys. Chem. A 109 (2005) 4359-4365. |