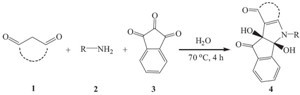
One of the powerful tools used to connect economic features with the green concerns is performing organic reactions in water; this strategy consists of two or more synthetic steps, which are carried out in water as a cheap, nontoxic, environmentally friendly solvent, in a one-step reaction, without isolation of any intermediate thus reducing time, saving money, energy and raw materials [1, 2, 3, 4, 5, 6, 7]. Indole moiety has been found in a wide variety of pharmacologically and biologically active compounds [8, 9]. For example, melatonin and indole-3-propionic acid (IPA) reduce reactive oxygen species that cause cellular damage and prevent death of neurons exposed to amyloid β-proteins, the agent responsible for Alzhimer’s disease [10]. Some indole derivatives function as dopamine agonists and/or selective serotonin reuptake inhibitors (SSRIs), the latter being a class of anti-depressants [11]. Acemetacin [12] and indometacin [13] are clinically used as antiinflammatory drugs and fluvastatin sodium [14] is a well-known HMG-CoA reductase inhibitor. Hence, we describe herein the reaction of cyclic-1,3-dione 1 and ninhydrin 3 in the presence of primary amines 2 in water as the green solvent.
All chemicals used in this work were purchased from Fluka (Buchs, Switzerland) and were used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H, and N were performed using a Heraeus CHN-O-Rapid analyzer. Mass spectra were recorded on a FINNIGAN-MAT 8430 spectrometer operating at an ionization potential of 70 eV. IR spectra were measured on a Shimadzu IR-460 spectrometer. 1H NMR and 13C NMRspectra were measured with a BRUKER DRX-500 AVANCE spectrometer at 500.1 and 125.8 MHz, respectively. 1H NMR and 13C NMR, spectra were obtained for solutions in CDCl3 using TMS as the internal standard or 85% H3PO4 as the external standard.
To a magnetically stirred solution of 1,3-activated dicarbonyl compound 1 (2mmol) and primary amine 2 (2mmol) in water (10mL) as the solventwas addedninhydrin 3 (2mmol).The reaction mixture was stirred for 4 h at 70 ℃. After completion of reaction (monitored by TLC), themixture of reactionwas purified in the room temperature by columnchromatography (silica gel (230-400 mesh, Merck) using n-hexane-EtOAc as eluent to give compound 4.
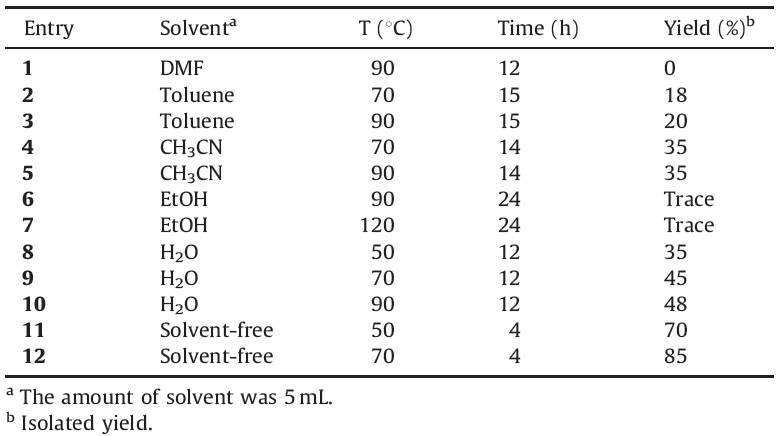
The starting point for our experiments was to optimize the reaction conditions such as solvent and reactions time for the production of indol derivatives which are usable in a wide variety of pharmacologically and biologically active compounds (Table 1).
| Table 1 Optimization of reaction conditions of compound 4a. |
|
Download:
|
| Scheme 1.Reaction of ninhydrin and 1,3-dione with primary amines. | |
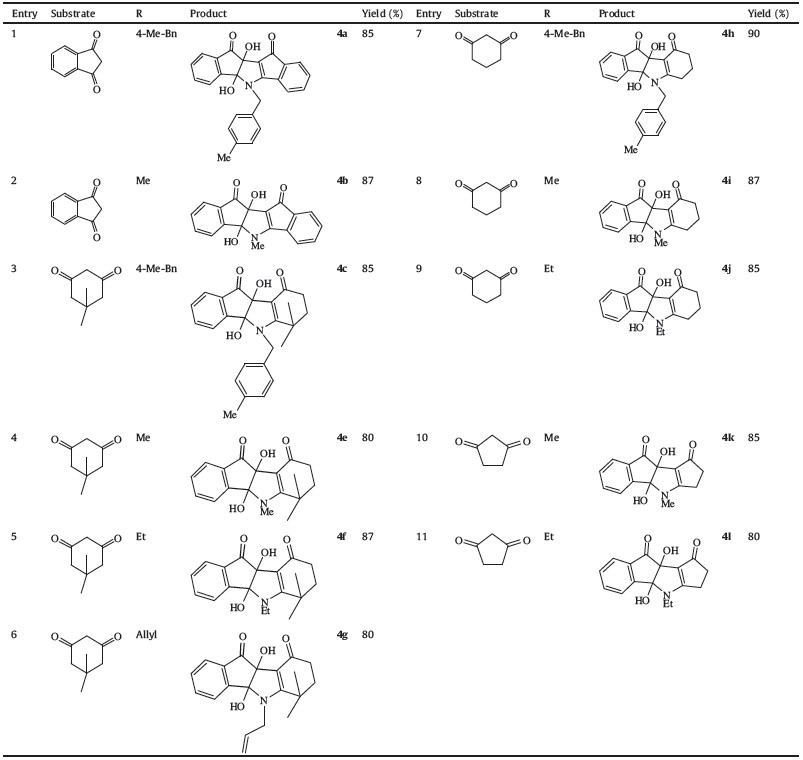
| Table 2 Functionalized hydroindoles. |
|
Download:
|
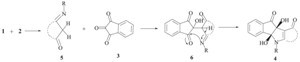
| Scheme 2.Proposed mechanism for the synthesis of compound 4. | |
In conclusion, we have described a convenient route to functionalized hydroindeno[1,2-b]indoles from a three-component reaction of ninhydrin, cyclic-1,3-dione and primary amines. The advantage of the present procedure is that the reaction is performed in water as the green solvent and by simple mixing of the starting materials. The procedure described here provides an acceptable one-pot method for the preparation of functionalized hydroindeno[1,2-b]indoles.
We gratefully acknowledge financial and spiritual support from Research Council of Gonbad Kavous University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2013.09.016.
| [1] | I. Ugi, Recent progress in the chemistry of multicomponent reactions, Pure Appl. Chem. 73 (2001) 187-191, and refrences there in. |
| [2] | J.P. Zhu, H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2005. |
| [3] | A. Domling, Multicomponent reactions, Chem. Rev. 106 (2006) 17-89. |
| [4] | C.C.A. Cariou, G.J. Clarkson, M. Shipman, Rapid synthesis of 1,3,4,4-tetrasubstituted β-lactams from methyleneaziridines using a four-component reaction, J. Org. Chem. 73 (2008) 9762-9764. |
| [5] | P. Anastas, T. Williamson, Green Chemistry, Frontiers in Benign Chemical Synthesis and Procedures, Oxford Science Publications, New York, 1998. |
| [6] | G.W.V. Cave, C.L. Raston, J.L. Scott, Recent advances in solventless synthesis with remarkable versatility, Chem. Commun. (2001) 2159-2169. |
| [7] | (a) R.A. Sheldon, Organic synthesis -past, present and future, Chem. Ind. (1992) 903-906; (b) R.A. Sheldon, Catalysis and pollution prevention, Chem. Ind. (1997) 12-15. |
| [8] | M.A. Pappolla,M. Sos, R.A. Omar, et al.,Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide, J. Neurosci. 17 (1997) 1683-1690. |
| [9] | Y.J. Chyan, B. Poeggeler, R.A. Omar, et al., Indole structure, indole-3-propionic acid, J. Biol. Chem. 274 (1999) 21937-21942. |
| [10] | T. Heinrich, H. Bottcher, A new synthesis of indole 5-carboxylic acids and 6-hydroxy-indole-5-carboxylic acids in the preparation of an o-hydroxylated metabolite of vilazodone, Bioorg. Med. Chem. Lett. 14 (2004) 2681-2684. |
| [11] | K.H. Boltze, O. Brendler, H. Jacobi, et al., The simultaneous anti-inflammatory and ulcerogenic action of non-steroidal Part II: gastric acid antisecretory and antiulcero-genic actions, Arzneim.-Forsch. 30 (1980) 1314-1325. |
| [12] | T.Y. Shen, T.B. Windholz, A. Rosegay, et al., Non-steroid anti-inflammatory agents, J. Am. Chem. Soc. 85 (1963) 488-489. |
| [13] | K. Hayashi, J. Kurokawa, S. Nomura, et al., The application of N G-nitro-L-arginine methyl ester (l-NAME), a NOS inhibitor, levels in homozygous Watanabe-heritable hyperlipidemic rabbits, Biochim. Biophys. Acta 1167 (1993) 223-225. |
| [14] | I. Yavari, M. Adib, M.H. Sayahi, An efficient diastereoselective one-pot synthesis of dihydrofuro[20,30:2,3]indeno[2,1-b]furan derivatives, Tetrahedron Lett. 43 (2002) 2927-2929. |