b Department of Chemistry, College of Sciences, Shahid Chamran University, Ahvaz 61357-4-3169, Iran
Suzuki-Miyaura C-C cross-coupling reaction catalyzed by palladium species is one of the most important synthetic transformations involved in synthesis of a large number of organic compounds [1, 2, 3, 4, 5, 6]. Reaction solvents as one of the most important constituents in any chemical process, including Suzuki-Miyaura coupling reactions, play an essential role in deciding its environmental impact as well as its cost, safety and health issues. Organic solvents are often toxic, highly flammable, volatile and non-renewable and have low heat capacities. In contrast, water is non-toxic and non-flammable, has a high heat capacity, and is relatively cheap, so it would appear to be an attractive solvent [7, 8, 9].
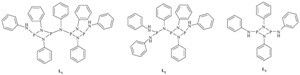
In general, improvements of palladium-catalyzed reactions are based largely on the reactivities of the palladium catalyst by using efficient supporting ligands [10, 11, 12]. Therefore, during the past decades many efforts have been made to find the most efficient ligands, in which phosphine-based ligands are used as the most common ligands for the Suzuki coupling reactions; however, most of the phosphine-based ligands are water and/or air-sensitive [13, 14]. Therefore, it is highly desirable to design water soluble, air, moisture, and thermally stable and efficient ligands for the palladium-catalyzed coupling reactions. Here, we report the Pdcatalyzed Suzuki reaction using some phosphazane derivatives, as ligands, which display good reactivity and stability (Scheme 1).
|
Download:
|
| Scheme 1.The phosphazane oligomers (L1–3) used as ligand in Suzuki reaction. | |
All chemicals were purchased as reagent grade from commercial suppliers and were used without further purification unless otherwise noted. Phosphazane derivatives (L1-3) were prepared according to the literature procedures published by our group [15, 16]. Solvents were dried by passing over Al2O3 and/or by storing over molecular sieves unless otherwise noted. The 1H NMR and 13C-NMR spectra were recorded on a Bruker FT-NMR 500 MHz spectrometer.
All Suzuki reactions were carried out without an inert atmosphere. A mixture of aryl halide (1.0 mmol), arylboronic acid (1.2 mmol), K2CO3 (2 mmol), Pd(OAc)2 (0.5 mol%) and phosphazane ligand (L1-3) (1.0mol%) in H2O (3 mL) was allowed to react in a sealed tube at 90 ℃. The reaction mixtures were added to brine (15 mL) and extracted three times with diethyl ether (3 × 15 mL). The further purification of the product was achieved by flash chromatography on a silica gel column using hexane/ethyl acetate (4:1).
Characterization data of ligands (L1-3) and coupling products are listed in Supporting information.
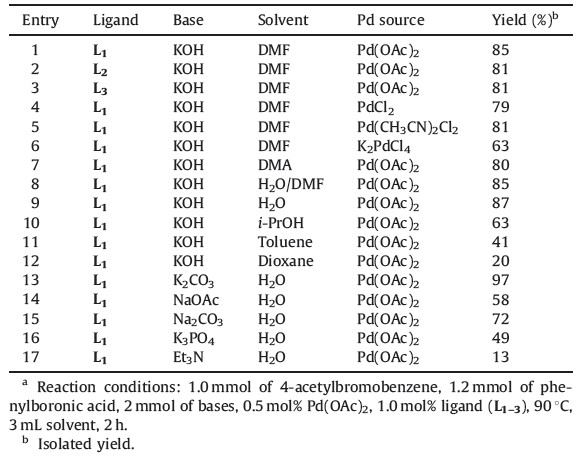
Phosphorus trichloride and PhNH2, react readily to form a series of PhNH/PhN-substituted P(Ⅲ) phosphazanes. According to our previous literature [15, 16], the nearly quantitative product L1 is obtained at 110 ℃ in toluene, with a PCl3:PhNH2 molar ratio of 1:4.8. Compound L2 is formed in greater than 80% yield by reaction of PCl3 with PhNH2 (1:6 molar ratio, 2 h, 0 ℃) in methylene chloride. Also at r.t.with a PCl3:PhNH2 molar ratio of 1:5, themain product L3 is produced. To investigate the reactivity of L1-3 as ligand, we first employed 4-acetylbromobenzene and phenylboronic acid as substrates to optimize the reaction conditions. Their coupling reactions were carried out under a variety of palladium sources, bases and solvents. The results are summarized in Table 1.
| Table 1 The optimized reaction condition for the Pd-catalyzed Suzuki reaction.a |
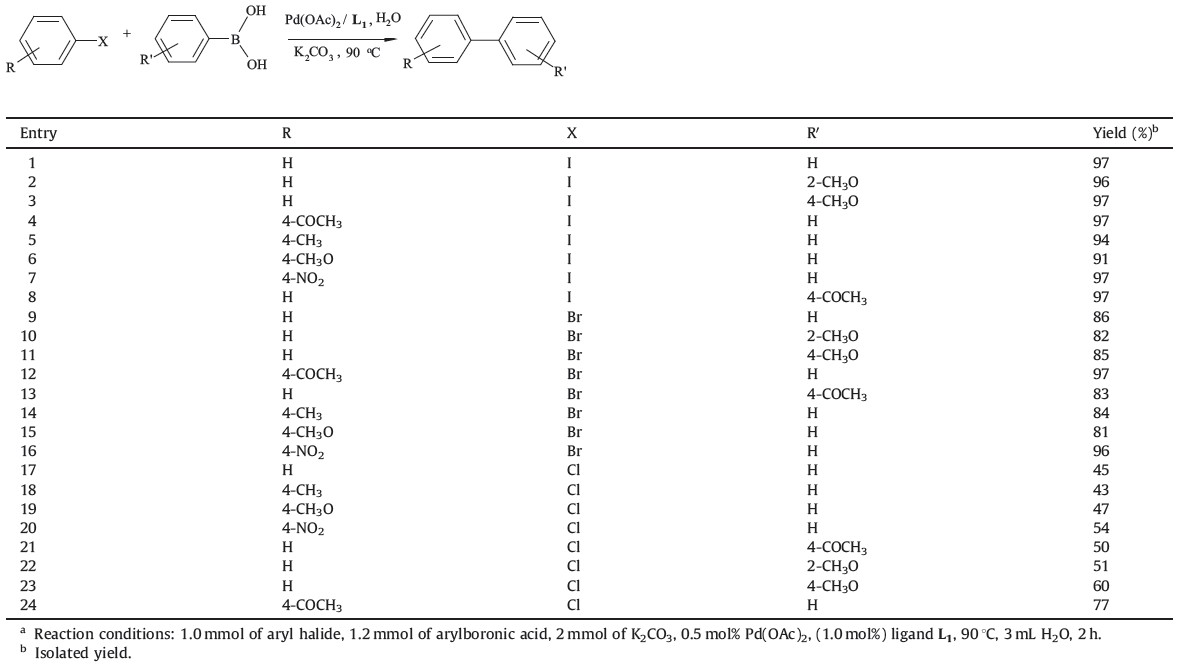
The catalytic applicability of the phosphazane ligand L1 was explored with a range of aryl halides and arylboronic acids with the results summarized in Table 2. All the reactions were carried out at 90 ℃ in air using H2O as a reaction solvent.
As shown in Table 2, the Suzuki coupling of various phenylboronic acids with aryl iodide and bromide provided good to excellent yields of the products (entries 1-16). The reactions between aryl iodides and various phenylboronic acids were quantitative and afforded biphenyl derivatives in 91%-97% yields within 2 h with the use of 0.5 mol% of Pd(OAc)2 in H2O. It is worth noting that the most electron-deficient aryl bromides, 4- acetyl and 4-nitro bromobenzene, quantitatively gave the coupling products (entries 12 and 16). In order to test the feasibility of this protocol for challenging substrates, we also conducted the Suzuki reactionswith aryl chlorides and observed moderate to good product yields (entries 17-21). High catalytic activity was observed in the coupling of phenylboronic acid and 4-methoxyphenylboronic acid with aryl iodides and bromides, and moderate to good activity with chlorides. 2-Methoxyphenylboronic acid was less reactive in comparison to both phenylboronic acid and 4-methoxy phenylboronic acid (entries 2, 10 and 22).
Recently, Iranpoor and co-workers reported the use of 1,3,2,4- diazadiphosphetidine compounds (L1-3) as ligands for Pd(Ⅱ)- catalyzed Suzuki coupling reactions of aryl iodides, bromides, and chlorides under base-free conditions in water [17]. In fact, they used high amounts of phosphazen ligands (0.3 mmol) as both a ligand and a base. According to high cost and toxicity of these ligands, use of a high amount of them is not good idea and therefore in this work we use only 1.0 mol% of phosphazane derivatives as ligand (0.01 mmol) for Suzuki coupling reaction. In contrast to similar, previously reported systems, the catalytic system presented in this paper does not suffer from the harsh reaction conditions, such as using large amounts of hazardous phosphine-based ligands, long reaction time and high reaction temperature, but both two systems are almost equally effective Suzuki coupling catalysts.
| Table 2 The Suzuki reaction between aryl halides and aryl boronic acids.a |
In conclusion, we have developed an efficient and environmentally friendly water-soluble phosphazane oligomer as ligand which is readily accessible from inexpensive and commercial materials. The in situ generated catalysts from Pd(OAc)2 and the phosphazane ligand L1 demonstrated good catalytic activities towards the Suzuki reaction of aryl halides and various phenylboronic acids in presence of water, as the green solvent. Under aerobic conditions, good to excellent yields of the coupled products were obtained.
M. Amini and A. Tarassoli thank respectively the Research Council of University of Maragheh and Shahid Chamran University for funding of this work.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2013.10.002.
| [1] | N. Miyaura, A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev. 98 (1995) 2457-2483. |
| [2] | S. Singha, M. Sahoo, K.M. Parida, Highly active Pd nanoparticles dispersed on amine functionalized layered double hydroxide for Suzuki coupling reaction, Dalton Trans. 40 (2011) 7130-7132. |
| [3] | M. Amini, M. Bagherzadeh, Z. Moradi-Shoeili, D.M. Boghaei, Pd(OAc)2 without added ligand as an active catalyst for Mizoroki-Heck reaction in aqueous media, RSC Adv. 2 (2012) 12091-12095. |
| [4] | Z.Z. Zhou, F.S. Liu, D.S. Shen, C. Tan, L.Y. Luo, Efficient palladium-catalyzed Suzuki cross-coupling reaction with β-ketoamine ligands, Inorg. Chem. Commun. 14 (2011) 659-662. |
| [5] | M. Amini, H. Etemadi, D-Glucosamine as an efficient and green additive for palladium-catalyzed Heck reaction, Chem. Pap. 67 (2013) 759-763. |
| [6] | M. Amini, M. Bagherzadeh, S. Rostamnia, Efficient imidazolium salts for palladium-catalyzed Mizoroki-Heck and Suzuki-Miyaura cross-coupling reactions, Chin. Chem. Lett. 24 (2013) 433-436. |
| [7] | H.D. Velazquezd, F. Verpoort, N-heterocyclic carbene transition metal complexes for catalysis in aqueous media, Chem. Soc. Rev. 41 (2012) 7032-7060. |
| [8] | C.J. Li, Organic reactions in aqueous media with a focus on carbon-carbon bond formations: a decade update, Chem. Rev. 105 (2005) 3095-3166. |
| [9] | K. Shaughnessy, Hydrophilic ligands and their application in aqueous-phase metal-catalyzed reactions, Chem. Rev. 109 (2009) 643-710. |
| [10] | S. Mohanty, D. Suresh, M.S. Balakrishna, J.T. Mague, An inexpensive and highly stable ligand 1, 4-bis(2-hydroxy-3,5-di-tert-butylbenzyl)piperazine for Mizoroki-Heck and room temperature Suzuki-Miyaura cross-coupling reactions, Tetrahedron 64 (2008) 240-247. |
| [11] | X.Q. Zhang, Y.P. Qiu, B. Rao, M.M. Luo, Palladium(Ⅱ)-N-heterocyclic carbene metallacrown ether complexes: synthesis, structure, and catalytic activity in the Suzuki-Miyaura reaction, Organometallics 28 (2009) 3093-3099. |
| [12] | B.P. Morgan, G.A. Galdamez, R.J. Gilliard Jr., R.C. Smith, Canopied trans-chelating bis(N-heterocyclic carbene) ligand: synthesis, structure and catalysis, Dalton Trans. (2009) 2020-2028. |
| [13] | N.G. Andersen, B.A. Keay, 2-Furyl phosphines as ligands for transition-metalmediated organic synthesis, Chem. Rev. 101 (2001) 997-1030. |
| [14] | R. Martin, S.L. Buchwald, Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands, Acc. Chem. Res. 41 (2008) 1461-1473. |
| [15] | M.L. Thompson, A. Tarassoli, R.C. Haltiwanger, A.D. Norman, Synthesis of two new1, 3,2,4-diazadiphosphetidine-based phosphazane oligomers:[(PhNH)P2(NPh)2]2NPh and [(PhNH)PNPh]3, Inorg. Chem. 26 (1987) 684-689. |
| [16] | M.L. Thompson, A. Tarassoli, R.C. Haltiwanger, A.D. Norman, Synthesis of [(C6H5NH)PNC6H5]3: a participant in a phosphorus(II1)-nitrogen compound trimer-dimer interconversion reaction, J. Am. Chem. Soc. 103 (1981) 6770-6772. |
| [17] | N. Iranpoor, H. Firouzabadi, A. Tarassoli, M. Fereidoonnezhad, 1, 3,2,4-Diazadiphosphetidines as ligand and base for palladium-catalyzed suzuki-miyaura, sonogashira-hagihara, and homocoupling reactions of aryl halides under heterogeneous conditions in water, Bull. Chem. Soc. Jpn. 83 (2010) 1367-1373. |