Diaryl ethers are very important organic compounds which have played a significant role in the production of fragrances [1, 2, 3, 4], biological materials [5, 6, 7, 8, 9] and pharmaceutical compounds [10, 11, 12]. The synthesis of diaryl ethers, therefore, has attracted enormous attention of synthetic organic chemists with a great number of methods having been developed. The conventional Ullmann cross-coupling reaction to form diary ethers [13, 14, 15] has been reported using aryl halides and phenols with copper or palladium as catalyst [16, 17, 18, 19, 20, 21]. However, this application was limited by harsh reaction conditions, such as high temperature [22, 23], excess quantities of copper reagents [24] and highly expensive ligands [25, 26, 27, 28]. Moreover, palladium is expensive compared to copper or copper complexes.
Although several researchers have investigated CuO as catalyst for the Ullmann cross-coupling reaction [29, 30], little work has been published on the simple method involving the recyclability of the catalyst. In 2011, an important breakthrough was achieved by Cano and co-workers [31, 32]. They reported the catalyst CuOFe3O4 can be used in the C-N coupling reaction and the different domino Sonogashira cyclization processes between 2-iodophenol and different alkynes with the catalyst very easily removed from the reaction mixture just by using a simple magnet. Herein, we report CuO-Fe3O4-catalyzed O-arylation of a wide range of aryl halides with various substituted phenols. To the best of our knowledge, this is the first report on the use of CuO-Fe3O4 in the formation of the C-O bond in the synthesis of diaryl ethers.
All reagents were purchased from Aladdin and were used without further purification unless otherwise noted. Analytical thin layer chromatography (TLC) was performed on silica G 60 F254 (0.25 mm) plates with visualization by UV light (254 nm and 365 nm). The 1H NMR spectra were recorded on Bruker Avance Digital (400 MHz) spectrometers with Me4Si as the internal standard. The catalyst CuO-Fe3O4 was prepared according to the previous research work [32].
The morphology of the catalyst was characterized by scanning electron microscopy (SEM, JCM-6700, Japan) and transmission electron microscopy (TEM, TECNAI G2 TF20). The surface composition of catalyst was detected by X-ray photoelectron spectra (XPS, PHI-5702).
To a stirred solution of phenol (2.2 mmol) in DMF (15.0 mL) under an argon atmosphere were added CuO-Fe3O4 (0.2 mmol), TBAB (0.2 mmol), Cs2CO3 (326 mg, 1.0 mmol) and aryl halide (2.0 mmol). The reaction mixture was stirred at the required temperature (145 ℃) for 24 h. At the end of reaction, the catalyst was removed by a magnet and the resulting mixture was quenched with water and extracted with EtOAc. The organic phases were dried over MgSO4, followed by evaporation under reduced pressure to remove the solvent. The residue was purified by column chromatography on silica gel to afford the desired product.
The microstructure and elemental composition of the catalyst are characterized by SEM and EDX microanalysis. Fig. 1(a) and (b) shows the SEM and EDX of CuO-Fe3O4. Fig. 1(a) shows that the catalyst is dense, implying the magnetic material exhibited poor dispersion. Fig. 1(b) indicates that the Cu, Fe, and O components are detected by EDX, and no impurities are observed. The inset table in Fig. 1(b) shows the measured elemental composition of the sample surface.

|
Download:
|
| Fig. 1.SEM (a) image and EDX (b) spectrum of CuO-Fe3O4. | |

|
Download:
|
| Fig. 2.TEM image of (a) CuO-Fe3O4 and (b) after third reuse of catalyst CuO-Fe3O4. | |
|
Download:
|
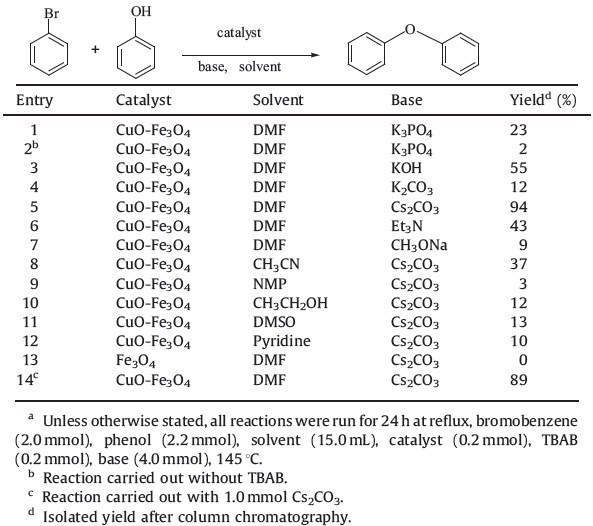
| Fig. 3.XPS spectra of sample CuO-Fe3O4: (a) survey spectrum, (b) Cu2p spectrum in CuO-Fe3O4. | |
| Table 1 Optimization of the reaction conditions.a |
| Table 2 CuO-Fe3O4-catalyzed O-arylation of phenols with aryl halides.a |
| Table 3 Recycle of catalyst.a |
|
Download:
|
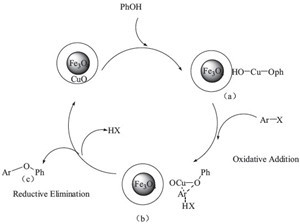
| Scheme 1.Plausible mechanism for the CuO-Fe3O4-catalyzed O-arylation of phenols with aryl halides. | |
In conclusion, we have developed a ligand-free, highly efficient, experimentally simple Ullmann cross-coupling reaction of phenols with aryl halides using impregnated copper on magnetite as catalyst. The optimal reaction conditions were determined as follows: phenol and bromobenzene in the presence of CuO-Fe3O4 as catalyst; DMF as solvent; TBAB as phase transfer catalysis; Cs2CO3 as base at 145 ℃. Moreover, the catalyst can be recovered by a simple magnet from the reaction mixture and reused 3 times with high catalytic activities.
This work was supported by the Natural Science Foundation of Gansu Province, China (No. 1014RJZA022) and Postgraduate Tutor Fund of Gansu Province Education Department (No. 1101ZTC103).
| [1] | E. Buck, Z.J. Song, D. Tschaen, et al., Ullmann diaryl ether synthesis: rate acceleration by 2,2,6,6-tetramethylheptane-3,5-dione, J. Org. Lett. 4 (2002) 1623-1626. |
| [2] | Y. Asakawa, Biologically active compounds from bryophytes, Pure Appl. Chem. 79 (2007) 557-580. |
| [3] | A.B. Naidu, G. Sekar, An efficient intermolecular BINAM-copper (Ⅰ) catalyzed Ullmann-type coupling of aryl iodides/bromides with aliphatic alcohols, Tetrahedron Lett. 49 (2008) 3147-3151. |
| [4] | M. Islam, S. Mondal, M. Manir, et al., Synthesis and characterization of polymer anchored Cu(Ⅱ) complexes: heterogeneous catalysts for preparation of diaryl ethers, Chin. J. Chem. 28 (2010) 1810-1820. |
| [5] | C.T. Helmes, C.C. Sigman, D.L. Atkinson, et al., A study of ethers for the selection of candidates for carcinogen bioassay, J. Environ. Sci. Health A 18 (1983) 797-839. |
| [6] | J. Zhu, SNAr based macrocyclization via diaryl ether formation: application in natural product synthesis, Synlett 2 (1997) 133-134. |
| [7] | T. Eicher, S. Fey, W. Puhl, et al., Syntheses of cyclic bisbibenzyl systems, Eur. J. Org. Chem. 1998 (1998) 877-888. |
| [8] | J.S. Freundlich, M. Yu, E. Lucumi, et al., Synthesis and biological activity of diaryl ether inhibitors of malarial enoyl acyl carrier protein reductase: Part 2. 20-Substituted triclosan derivatives, Bioorg. Med. Chem. Lett. 16 (2006) 2163-2169. |
| [9] | J.S. Freundlich, J.W. Anderson, D. Sarantakis, et al., Synthesis, biological activity, and X-ray crystal structural analysis of diaryl ether inhibitors of malarial enoyl acyl carrier protein reductase: Part 1. 40-Substituted triclosan derivatives, Bioorg. Med. Chem. Lett. 15 (2005) 5247-5252. |
| [10] | J. Niu, H. Zhou, Z. Li, et al., An efficient Ullmann-Type C-O bond formation catalyzed by an air-stable copper(Ⅰ)-bipyridyl complex, J. Org. Chem. 73 (2008) 7814-7817. |
| [11] | A. Aranyos, W. David, A. Kiyomori, et al., Novel electron-rich bulky phosphine ligands facilitate the palladium-catalyzed preparation of diaryl ethers, J. Am. Chem. Soc. 121 (1999) 4369-4378. |
| [12] | K.W. Anderson, T. Ikawa, R.E. Tundel, et al., The selective reaction of aryl halides with KOH: synthesis of phenols, aromatic ethers, and benzofurans, J. Am. Chem. Soc. 128 (2006) 10694-10695. |
| [13] | F. Ullmann, Ueber eine neue Darstellungsweise von Phenyläthersalicylsäure, Ber. Dtsch. Chem. Ges. 37 (1904) 853-857. |
| [14] | A. Ouail, J.F. Spindler, A. Jutand, et al., Nitrogen ligands in copper-catalyzed arylation of phenols: structure/activity relationships and applications, Adv. Synth. Catal. 349 (2007) 1906-1916. |
| [15] | J.S. Sawyer, Recent advances in diaryl ether synthesis, Tetrahedron 56 (2000) 5045-5065. |
| [16] | D.D. Hennings, T. Iwama, V.H. Rawal, Palladium-catalyzed (Ullmann-type) homocoupling of aryl halides: a convenient and general synthesis of symmetrical biaryls via inter-and intramolecular coupling reactions, Org. Lett. 1 (1999) 1205-1208. |
| [17] | B. Yuan, Y. Pan, Y. Li, et al., A highly active heterogeneous palladium catalyst for the Suzuki-Miyaura and Ullmann coupling reactions of aryl chlorides in aqueous media, Angew. Chem. Int. Ed. 49 (2010) 4054-4058. |
| [18] | I.P. Beletskaya, A.V. Cheprakov, Copper in cross-coupling reactions: the post-Ullmann chemistry, Coord. Chem. Rev. 248 (2004) 2337-2364. |
| [19] | N. Xia, M. Taillefer, Copper-or iron-catalyzed arylation of phenols from respectively aryl chlorides and aryl iodides, Chem. Eur. J. 14 (2008) 6037-6039. |
| [20] | W.J. Liu, D.S. Mei, W.H. Duan, Synthesis of 1,7-dimethoxy-2-hydroxyxanthone, a natural product with potential activity on erectile dysfunction, Chin. Chem. Lett. 24 (2013) 515-517. |
| [21] | X.C. Yu, B. Li, B.H. Yu, et al., Efficient synthesis of unsymmetrical diaryl thioethers via TBAF-mediated denitrative substitution of nitroarenes with PhSTMS under mild and neutral conditions, Chin. Chem. Lett. 24 (2013) 605-608. |
| [22] | F. Monnier, M. Taillefer, Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions: copper makes a difference, Angew. Chem. Int. Ed. 47 (2008) 3096-3099. |
| [23] | M. Kidwai, N.K. Mishra, V. Bansal, et al., Cu-nanoparticle catalyzed O-arylation of phenols with aryl halides via Ullmann coupling, Tetrahedron Lett. 48 (2007) 8883-8887. |
| [24] | P.X. Ling, D. Li, X.Y. Wang, Supported CuO/γ-Al2O3 as heterogeneous catalyst for synthesis of diaryl ether under ligand-free conditions, J. Mol. Catal. A: Chem. 357 (2012) 112-116. |
| [25] | J. Hassan, M. Sevignon, C. Gozzi, et al., Aryl-aryl bond formation one century after the discovery of the Ullmann reaction, Chem. Rev. 102 (2002) 1359-1470. |
| [26] | S.A.R. Mulla, S.M. Inamdar, M.Y. Pathan, et al., Ligand free, highly efficient synthesis of diaryl ether over copper fluorapatite as heterogeneous reusable catalyst, Tetrahedron Lett. 53 (2012) 1862-1869. |
| [27] | A.K. Verma, Benzotriazole and its derivatives as ligands in coupling reaction, Adv. Heterocycl. Chem. 107 (2012) 101-132. |
| [28] | S.L. Yang, W.B. Xie, H. Zhou, et al., Alkoxylation reactions of aryl halides catalyzed by magnetic copper ferrite, Tetrahedron 69 (2013) 3415-3418. |
| [29] | J. Zhang, J. Chen, M. Liu, Ligand-free copper-catalyzed coupling of nitroarenes with arylboronic acids, Green Chem. 14 (2012) 912-916. |
| [30] | S.G. Babu, R. Karvembu, Room temperature Ullmann type C-O and C-S cross coupling of aryl halides with phenol/thiophenol catalyzed by CuO nanoparticles, Tetrahedron Lett. 54 (2013) 1677-1680. |
| [31] | R. Cano, D.J. Ramó n, M. Yus, Impregnated ruthenium on magnetite as a recyclable catalyst for the N-alkylation of amines, sulfonamides, sulfinamides, and nitroarenes using alcohols as electrophiles by a hydrogen autotransfer process, J. Org. Chem. 76 (2011) 5547-5557. |
| [32] | R. Cano, M. Yus, D.J. Ramó n, Impregnated copper or palladium-copper on magnetite as catalysts for the domino and stepwise Sonogashira-cyclization processes: a straightforward synthesis of benzo[b]furans and indoles, Tetrahedron 68 (2012) 1393-1400. |
| [33] | F.F. Jin, Y. Shen, R. Wang, et al., Double-perovskite PrBaCo2/3Fe2/3Cu2/3O5+δ as cathode material for intermediate temperature solid-oxide fuel cells, J. Power Sources 234 (2013) 244-251. |
| [34] | S. Poulston, P.M. Parlett, P. Stone, et al., Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES, Surf. Interface Anal. 24 (1996) 811-820. |