b College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, China
Many chemical processes are carried out in homogeneous solutions in order to increase the rate of reaction. However, most solvents used for reactions are volatile organic compounds (VOC), which easily evaporate into the atmosphere with detrimental effects on the environment and human health. Room-temperature ionic liquids, a type of "green solvent" with low viscosity and no measurable vapour pressure, have been developed to overcome these obstacles of traditional solvents [1, 2, 3]. Deep eutectic solvents (DES), a type of ionic solvent, has also been utilized for a long time due to their interesting solvent properties, such as dissolution of inorganic metal compounds [4, 5, 6, 7]. The first reported deep eutectic phenomenon [6] was a mixture of choline chloride (as organic salt) and urea (as hydrogen bond donor) in a 1:2 molar ratio, melting as low as 12 ℃. The mixture of ZnCl2 with choline chloride (ChCl) at a molar ratio of 1:2 was also reported with Tf of 23 ℃ [8]. Another type of room temperature DES is formed by amides and ZnCl2 (or AlCl3) through the disproportionation of ZnCl2 to form [ZnCl]+ and [ZnCl3]- [5]. Although the freezing points of acetamide-AlCl3 (molar ratio = 1:1) is very low at -63 ℃ [9], acetamide-AlCl3 is very sensitive to moisture and the preparation conditions are extremely strict. A series of ternary deep eutectic solvents made from sugar-urea-salt mixtures were also reported as solvents for organic synthesis [10, 11].
It has been reported that the freezing point of a deep eutectic solvent is partially determined by the melting points of the pure constituent parts and also related to the interaction between the cation and anion component [12]. Moreover, the viscosity and conductivity of DESs are also affected by the size of the ionic species and the voids in the liquid [7]. Recently, we found that alkylimidazolium halides ([BMIM]X, X = Cl, Br), zinc halides (ZnX2, X = Cl, Br) and amides formed lower freezing point ternary deep eutectic solvents (TDESs) in appropriate molar ratios than conventional DESs. Depending on the activity of the cation, the Lewis acid activity and coordinative properties of the large anion formed by zinc halide and amide, the TDESs can be used as both the solvent and catalyst in many organic reactions. The freezing point, density, viscosity and the electrical conductivity of TDESs with varying anions and amides were measured to establish their structure-property relationships.
1-Butyl-3-methyl imidazolium chloride ([BMIM]Cl, 99%, J&K) and 1-butyl-3-methyl imidazolium bromide ([BMIM]Br, 99%, J&K) were freeze dried at -55 ℃ for 12 h. ZnCl2 (AR, Sinopharm), ZnBr2 (99.9%, Aladdin), acetamide (AR, Sinopharm), N,N'-dimethylurea (98%, Sinopharm) and urea (AR, Sinopharm) were dried at 60 ℃ for 24 h under vacuum.
TDESs were prepared by heating the mixtures of the corresponding reagents with the required molar ratio at the appropriate temperature (around 100 ℃) for 2-4 h. A homogeneous liquid was obtained and then allowed to be cooled to room temperature. All products were dried under vacuum at 80 ℃ for 24 h before characterization.
The freezing points of TDESs were determined with a differential scanning calorimeter (DSC Perkin Elmer TA MDSC2910, USA), by sealing ca. 10 mg of the sample in an aluminum pan. The pan and sample were first cooled to about -80 ℃ with liquid nitrogen and then heated to 100 ℃ at a rate of 10 K/min. Densities of TDESs were determined only at 300.2 K using a KEM-DA100 vibrating tube density/specific gravity meter (uncertainty ±0.0003 g/cm3, Kyoto Electronics Manufacturing Co., Ltd.). To check the density meter adjustment, density of water (degassed distillated) was measured at 300.2 K. The kinetic viscosity measurement was measured in a rotating viscometer (Model NDJ-79, Shanghai, China) using a standard method (ASTM D2162-06) at T = 298.2 K. The ionic conductivity measurement was performed by using a conductivity meter (FE30, Mettler-Toledo Group, Shanghai, China) within 298.2- 333.2 K in 5 K increments. The instrument was first calibrated with 0.0100 mol/L standard KCl aqueous solution at every measurement temperature.
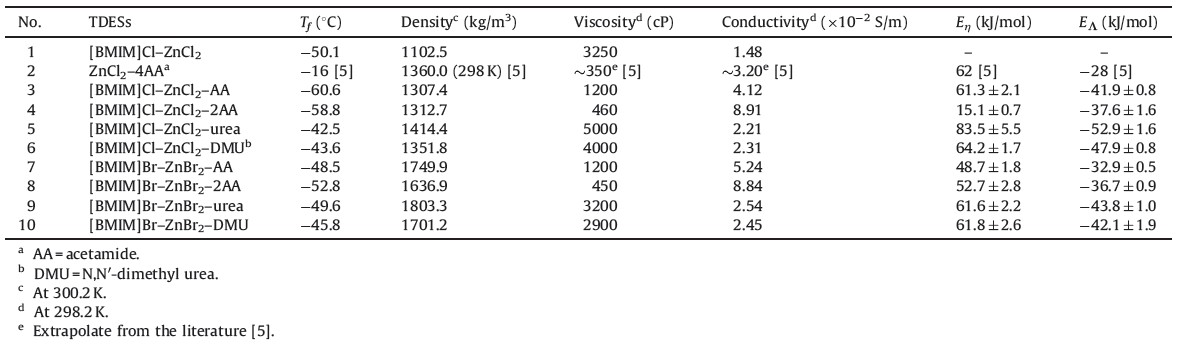
The ternary deep eutectic solvents were prepared by heating the mixtures of [BMIM]X, zinc halides (ZnX2) and amides with different molar ratios at the appropriate temperature (around 100 ℃). When a homogeneous liquid was obtained, the mixture was gradually cooled down to room temperature. Among the novel ternary DES systems, [BMIM]Cl-ZnCl2-AA (AA = acetamide) systems were found to have the lowest freezing points (-60.6 ℃) and can be used as ambient temperature solvents, which are stable for months. This freezing point is considerably lower than that of either of the components (mp [BMIM]Cl = 65 ℃, ZnCl2 ≈ 290 ℃ [13] and acetamide = 80 ℃), and also lower than the typical binary deep eutectic solvents (mp. ZnCl2-acetamide (1:4) = -16 ℃ [5] and [BMIM]Cl-ZnCl2 = -50.1 ℃, measured in this investigation). The [BMIM]Cl-ZnCl2-2AA system was also examined by differential scanning calorimetry (DSC), undergoing a glass transition around -58.8 ℃ (Table 1), andwas also stable for months. However, for the system with the molar ratio of acetamide (AA) exceeding 2 to another two components, a homogeneous liquid can only be formed in the heating process (>100 ℃). After cooling, those systems slowly become waxy solid or unstable liquid-solid mixtures at ambient temperature. Acetamide needle crystals were observed inside and confirmed by FTIR spectra.
| Table 1 The freezing point (Tf), density, viscosity, conductivity, Eη and EΛ of TDESs. |
The results also showed that the type of halide anion governs the physical properties of TDES. Cl- has a greater capability of coordination with Zn2+ and stronger hydrogen bond interactions with amides than Br-. As a result, Coulombic interactions between the complex anion and the alkyl-substituted imidazolium cation are different between Cl-systems and Br-systems. It is shown that the depression of freezing point decreases in the order Cl- > Br-.
As shown in Table 1, the addition of amides in the system [BMIM]Cl-ZnCl2-AA increased the density of [BMIM]Cl-ZnCl2. This could be due to the formation of complex anion [(ZnCl3(AA)]- or [ZnCl2(AA)2] (for molar ratio 1:1:2) between the carbonyl group and Zn2+ cation [14] decreasing the electrostatic repulsive-force between the [ZnCl3]- anions in [BMIM]Cl-ZnCl2. Although species of amides does not significantly influence the density of TDESs (Table 1), the acetamide-system TDESs generally show lower densities than the urea-system (including DMU) TDESs with the exception of [BMIM]Br-ZnBr2-AA. The results in Table 1 indicated that the density is also mainly governed by the halide anion in inorganic or organic salts. The densities of the Cl-system TDESs cover a density range from 1307.4 kg/m3 to 1414.4 kg/m3, while the Br-system TDESs cover a density range from 1636.9 kg/m3 to 1803.3 kg/m3. The corresponding Br-system TDESs display higher density values than that of Cl-system TDESs, which could be due to the increased size of the complex anions.
All TDESs are optically transparent and fluid. The viscosity values, ranging from 450 cP to 5000 cP (Table 1), are mostly lower than that of [BMIM]Cl [15]. For the ZnCl2-system TDES, the addition of acetamide (AA) significantly decreased the viscosity (for [BMIM]Cl-ZnCl2-2AA, 460 cP), as compared with [BMIM]Cl- ZnCl2 (3250 cP). According to the freezing point and viscosity of TDESs, viscosity is related to the type of amide. The viscosity increased in the order 2AA < AA < DMU < urea. This can be explained by the capability of coordination of the amide with Zn2+. The formation of self-hydrogen bonds between amides can explain the high viscosity of urea-systems. The viscosities of the TDESs change significantly as a function of temperature and follow the Arrhenius model [16] at temperatures from 298.2 K to 333.2 K. The Eη (Table 1) for ternary DESs are also similar to conventional ionic liquids and binary DESs [5, 17].
The electrical conductivities of TDESs cover a conductivity range from 2.21 × 10-2 S/m to 8.91 × 10-2 S/m (Table 1). Since the conductivity pattern of TDESs is governed by their ion mobility, the conductivity should increase with the decrease of their viscosities [18]. As expected, both the conductivity of [BMIM]Cl-ZnCl2-AA and [BMIM]Cl-ZnCl2-2AA are higher than that of binary DESs ([BMIM]Cl-ZnCl2 and ZnCl2-acetamide (1:4)). The conductivity of TDESs decreased in the order 2AA > AA > DMU > urea. For every TDES, the decrease of viscosity with increasing of temperature results from an increase of the mobility of the ions and further conductivity of the TDESs. An exponential increasing of conductivity with increasing temperature was observed and can also be fitted by the Arrhenius equation (EΛ in Table 1).
A series of novel room-temperature ternary deep eutectic solvents based on imidazolium ionic liquids, zinc halides and amides were successfully prepared. Besides the low freezing points, the lower viscosity and higher conductivity of TDES make it suitable for promoting polar reactions. Moreover, the presence of a Lewis acidic center in TDESs using zinc halides makes it possible to be used both as catalyst and solvent in various catalytic reactions.
The authors would like to thank the Fundamental Research Funds for the Central Universities (No. 13D110520 and 13D310508) for funding this work.
| [1] | R.D. Rogers, K.R. Seddon, Ionic liquids -solvents of the future? Science 302 (2003) 792-793. |
| [2] | H.Z. Hui, L.S. Hong, H. Yun, et al., Epoxy ether cleaving reactions catalyzed by supporting Lewis acidic ionic liquid, Chin. Chem. Lett. 23 (2012) 1217-1220. |
| [3] | L. Zhang, M.H. Ding, Y.G. Hong, One-step synthesis of a,b-unsaturated arylsulfones by a novel multicomponent reaction of aromatic aldehydes, chloroacetonitrile, benzenesulfinic acid sodium salt, Chin. Chem. Lett. 23 (2012) 1352-1354. |
| [4] | Q.H. Zhang, V.K. De Oliveira, S. Royer, et al., Deep eutectic solvents: syntheses, properties and applications, Chem. Soc. Rev. 41 (2012) 7108-7146. |
| [5] | A.P. Abbott, J. Barron, K. Ryder, et al., Eutectic-based ionic liquids with metalcontaining anions and cations, Chem. Eur. J. 13 (2007) 6495-6501. |
| [6] | A.P. Abbott, G. Capper, D.L. Davies, et al., Novel solvent properties of choline chloride/urea mixtures, Chem. Commun. (2003) 70-71. |
| [7] | A.P. Abbott, G. Capper, S. Gray, Design of improved deep eutectic solvents using hole theory, ChemPhysChem 7 (2006) 803-806. |
| [8] | A.P. Abbott, G. Capper, D.L. Davies, et al., Ionic liquids based upon metal halide/ substituted quaternary ammonium salt mixtures, Inorg. Chem. 43 (2004) 3447-3452. |
| [9] | H.M.A. Abood, A.P. Abbott, A.D. Ballantyne, et al., Do all ionic liquids need organic cations?. Characterisation of [AlCl2 nAmide]+AlCl4 and comparison with imidazolium based systems, Chem. Commun. 47 (2011) 3523-3525. |
| [10] | G. Imperato, E. Eibler, J. Niedermaier, et al., Low-melting sugar-urea-salt mixtures as solvents for Diels-Alder reactions, Chem. Commun. (2005) 1170-1172. |
| [11] | G. Imperato, S. Hoger, D. Lenoir, et al., Low melting sugar-urea-salt mixtures as solvents for organic reactions -estimation of polarity and use in catalysis, Green Chem. 8 (2006) 1051-1055. |
| [12] | A.P. Abbott, D. Boothby, G. Capper, et al., Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids, J. Am. Chem. Soc. 126 (2004) 9142. |
| [13] | The Merck Index, 12th ed Entry# 10261. |
| [14] | V. Lecocq, A. Graille, C.C. Santini, et al., Synthesis and characterization of ionic liquids based upon 1-butyl-2,3-dimethylimidazolium chloride/ZnCl2, New J. Chem. 29 (2005) 700-706. |
| [15] | Q.L. Kuang, J. Zhang, Z.G. Wang, Revealing long-range density fluctuations in dialkylimidazolium chloride ionic liquids by dynamic light scattering, J. Phys. Chem. B 111 (2007) 9858-9863. |
| [16] | C. Comminges, R. Barhdadi, M. Laurent, et al., Determination of viscosity, ionic conductivity, and diffusion coefficients in some binary systems: ionic liquid + -molecular solvents, J. Chem. Eng. Data 51 (2006) 680-685. |
| [17] | A.P. Abbott, G. Capper, D.L. Davies, et al., Ionic liquid analogues formed from hydrated metal salts, Chem. Eur. J. 10 (2004) 3769-3774. |
| [18] | D. Agostino, R.C. Harris, A.P. Abbott, et al., Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy, Phys. Chem. Chem. Phys. 13 (2011) 21383-21391. |