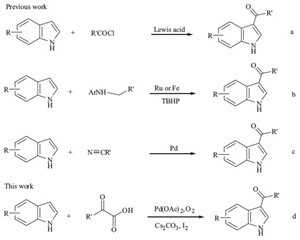
The indole ring systemrepresents one class of the most abundant and ubiquitous heterocycles in nature. Owing to their structural diversity and remarkable biological functions, the synthesis and transformation of indoles has been and continues to be a topic of research interest for synthetic organic chemists [1, 2, 3]. 3-Acylindoles are versatile intermediates in the syntheses of awide range of indole derivatives since their carbonyl groups can readily undergo a variety of transformations such as C-C and C-N coupling reactions and reductions [4, 5, 6]. Thus, the synthesis of 3-acylindoles has gained considerable attention. Traditionally, the common method for the preparation of 3-acylindoles is the Friedel-Crafts reaction (Scheme 1a) [7, 8, 9]. The other significant approaches include the Vilsmeier- Haack type reaction [10], and Grignard reactions [11]. Other methods involve nitrilium salts with dialkyl carbenium ions and pyridinium salts. Under the acidic conditions of Friedel-Crafts reactions, indole polymerization readily occurs. Vilsmeier-Haack acylindoles useunacceptable amounts of environmentallyunfriendly POCl3. The reactions of indole salts with acyl chlorides cannot tolerate the functional group sensitivities to strong nucleophiles because Grignard reagents are used in these reactions. The existing methods often involve uncommonly used acylated reagents or unbenign reaction conditions. As a consequence, the demand for alternative efficient methods has encouraged the development of newmethods for the synthesis of 3-acylindoles. In 2011, Su and coworkers reported a convenient and general method for formylation and acylation of free (N-H) indoles via Ru- or Fe- catalyzed oxidative coupling using anilines as the carbonyl source (Scheme 1b) [1]. Recently, Song and Wang independently reported the synthesis of 3-acylindoles through palladium-catalyzed addition of indoles to nitriles (Scheme 1c) [12, 13].
|
Download:
|
| Scheme 1.Existing routes and our strategy to 3-acylindoles. | |
Reagents were obtained commercially and used as received. Solvents were purified and dried by standard methods. The melting points were determined on an XT-4 micro melting point apparatus and uncorrected. IR spectra were recorded on an EQUINOX-55 spectrometer on a KBr matrix. NMR spectra were recorded on an INOVA-400 NMR instrument at room temperature using TMS as internal standard. Coupling constants (J) were measured in Hz. Chemical shift values (δ) are given in ppm. High Resolution mass spectrometer (HRMS) spectra were recorded on a Bruker micrOTOF-Q Ⅱ analyzer. A 200-300 mesh silica gel was used for column chromatography.
Representative procedure for the synthesis of 3-acylindoles (3): A 10 mL oven-dried Schlenk tube was charged with phenylglyoxylic acid 1a (49.5 mg, 0.33 mmol), indole 2a (35.1 mg, 0.3 mmol), I2 (164.5 mg, 0.65 mmol), Pd(OAc)2 (10 mol%, 6.7 mg, 0.03 mmol), Cs2CO3 (292 mg, 0.9 mmol). The tube was evacuated and filled with O2 (this procedure was repeated three times). Then NMP (1.5 mL) were added with a syringe under a counter flow of O2. The tube was sealed with a screw cap. The reaction was stirred at 45 ℃ for 12 h, and was then allowed to cool to ambient temperature. The mixture was added 20 mL EtOAc, and filtered, washed with water. The organic layers were dried over Na2SO4 and filtered. Solvents were evaporated under reduced pressure. The residue was purified by flash column chromatography with hexane/ethyl acetate to give the corresponding product 3.
(5,6-Dimethyl-1H-indol-3-yl)(phenyl)methanone (3af): Gray solid; Mp 192-194 ℃; IR (KBr, cm-1): vmax 3109, 2921, 1724, 1521, 1104; 1H NMR (400 MHz, DMSO-d6): δ 8.23 (s, 1H), 7.92 (s, 1H), 7.82-7.79 (m, 2H), 7.49-7.43 (m, 3H), 7.23 (s, 1H), 2.33 (s, 3H), 2.31 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 189.5, 137.8, 137.3, 136.2, 133.0, 132.3, 129.4, 128.6, 127.5, 120.8, 117.4, 113.4, 110.7, 20.5 20.3; HRMS (ESI) calcd. for C17H16NO (M+H)+ (m/z): 250.1227, found: 250.1231.
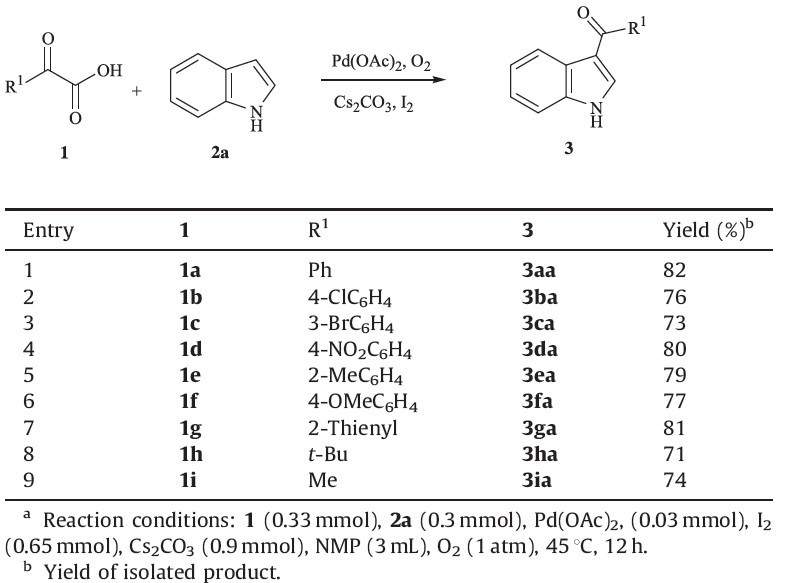
Our initial study started from the coupling of indole 2a with phenylglyoxylic acid 1a using O2 as the oxidant, Pd(TFA)2 as the catalyst in the presence of I2, K2CO3 and N,N-dimethylformamide (DMF) at 45 ℃ for 12 h. To our delight, the desired product 3aa was obtained in 43% yield (entry 1, Table 1). It was found that Pd(OAc)2 was superior to other Pd sources (entries 1-4, Table 1). Further studies indicated that the presence of iodine could promote the efficiency of this transformation (entry 5, Table 1). It was found that Cs2CO3 was superior to other bases (entries 4 and 6-8, Table 1). The influence of solvent on the reaction efficiency was also significant; when N-methylpyrrolidone (NMP) was chosen as the solvent, the yield was enhanced to 82% (entries 8-13, Table 1).
| Table 1 Palladium-catalyzed decarboxylative acylation of free (N–H) indoles.a |
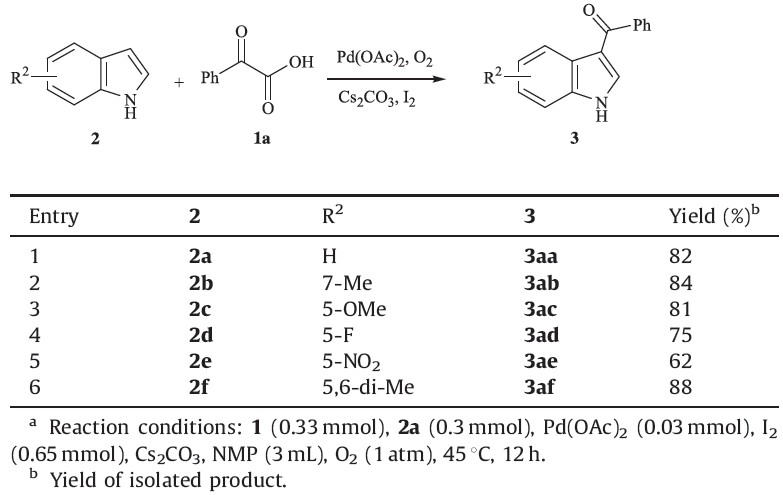
| Table 2 Palladium-catalyzed decarboxylative acylation of α-oxocarboxylic acids 1 with 2a.a |
| Table 3 Palladium-catalyzed decarboxylative acylation of α-oxocarboxylic acids 1a with 2.a |
|
Download:
|
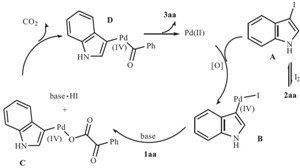
| Scheme 2.Proposed reaction mechanism. | |
In summary, we have developed a convenient and general method for acylation of free (N-H) indoles via a palladiumcatalyzed decarboxylative cross-coupling reaction. This process provided a useful method for the preparation of diverse 3- acylindoles in high yields from readily accessible reactants under mild reaction conditions. This protocol offers several advantages including use of readily accessible carboxylic acids, a simple procedure, generality, which enhances its potential in future applications.
We are grateful for the financial support from the State Ethnic Affairs Commission (No. 12YNZ005).
| [1] | W. Wu, W. Su, Mild and selective Ru-catalyzed formylation and Fe-catalyzed acylation of free (N-H) indoles using aniline as carbonyl source, J. Am. Chem. Soc. 133 (2011) 11924-11927. |
| [2] | Q. Yang, C. Xiao, L. Lu, et al., Synthesis of indoles through highly efficient cascade reaction of sulfur ylides and N-(ortho-chloromethyl)aryl amides, Angew. Chem. Int. Ed. 51 (2012) 9137-9140. |
| [3] | S.V. Goswami, P.B. Thorat, V.N. Kadam, S.A. Khiste, S.R. Bhusare, A convenient onepot three component synthesis of 3-aminoalkylated indoles catalyzed by 3-chlorophenylboronic acid, Chin. Chem. Lett. 24 (2013) 422-424. |
| [4] | H. Gadegoni, S. Manda, Synthesis and screening of some novel substituted indoles contained 1, 3,4-oxadiazole and 1,2,4-triazole moiety, Chin. Chem. Lett. 24 (2013) 127-130. |
| [5] | R. Lauchli, K. Shea, A synthesis of the Welwistatin core, Org. Lett. 8 (2006) 5287-5289. |
| [6] | S. Tohyama, T. Choshi, K. Matsumoto, A. Yamabuki, K. Ikegata, J. Nobuhiro, S. Hibino, A new synthesis of an indolo[3,2-j]phenanthridine alkaloid calothrixin B, Tetrahedron Lett. 46 (2005) 5263-5264. |
| [7] | N. Wan, Y. Hui, Z. Xie, J. Wang, Friedel-Crafts alkylation of indoles with nitroalkenes catalyzed by Zn(Ⅱ)-thiourea complex, Chin. J. Chem. 30 (2012) 311-315. |
| [8] | T. Watanabe, A. Kobayashi, M. Nishiura, et al., Synthetic studies on indoles and related compounds: XXVI. The debenzylation of protected indole nitrogen with aluminum chloride II, Chem. Pharm. Bull. 39 (1991) 1152-1156. |
| [9] | J.H. Wynne, C.T. Lloyd, S.D. Jensen, S. Boson, W.M. Stalick, 3-Acylindoles via a onepot, regioselective Friedel-Crafts reaction, Synthesis 14 (2004) 2277-2282. |
| [10] | W. Anthony, Novel synthesis of heterocyclic ketones, J. Org. Chem. 25 (1960) 2049-2053. |
| [11] | J. Bergman, L. Venemalm, Intramolecular, ring closure of α,β-unsaturated 3-acylindoles, Tetrahedron Lett. 28 (1987) 3741-3744. |
| [12] | T. Jiang, G. Wang, Synthesis of 3-acylindoles by palladium-catalyzed acylation of free (N-H) indoles with nitriles, Org. Lett. 15 (2013) 788-791. |
| [13] | Y. Ma, J. You, F. Song, Facile access to 3-acylindoles through palladium-catalyzed addition of indoles to nitrile: the one-pot synthesis of indenoindolones, Chem. Eur. J. 19 (2013) 1189-1193. |
| [14] | L.J. Goossen, G. Deng, L.M. Levy, Synthesis of biaryls via catalytic decarboxylative coupling, Science 313 (2006) 624-662. |
| [15] | N. Rodríguez, L.J. Goossen, C. Linder, Decarboxylative coupling reactions: a modern strategy for C-C bond formatiom, Chem. Soc. Rev. 40 (2011) 5030-5048. |
| [16] | R. Shang, Y. Fu, J. Li, et al., Synthesis of aromatic esters via Pd-catalyzed decarboxylative coupling of potassium oxalate monoesters with aryl bromides and chlorides, J. Am. Chem. Soc. 131 (2009) 5738-5739. |
| [17] | L.J. Goossen, F. Rudolphi, C. Oppel, N. Rodríguez, Synthesis of ketones from aoxocarboxylates and aryl bromides by Cu/Pd-catalyzed decarboxylative crosscoupling, Angew. Chem. Int. Ed. 47 (2008) 3043-3045. |
| [18] | P. Fang, M. Li, H. Ge, Room temperature palladium-catalyzed decarboxylative ortho-acylation of acetanilides withα-oxocarboxylic acids, J. Am. Chem. Soc. 132 (2010) 11898-11899. |
| [19] | L. Gu, C. Jin, J. Guo, L. Zhang, W. Wang, A novel strategy for the construction of substituted benzoxazoles via a tandem oxidative process, Chem. Commun. (2013), http://dx.doi.org/10.1039/c3cc46375c. |
| [20] | L. Gu, C. Jin, Synthesis and antitumor activity of α-aminophosphonates containing thiazole[5,4-b]pyridine moiety, Org. Biomol. Chem. 10 (2012) 7098-7102. |
| [21] | L. Gu, R. Wang, X. Huang, C. Jin, Novel synthetic route to α-aminophosphonates containing benzothiazole moiety, Chin. J. Chem. 30 (2012) 2483-2487. |
| [22] | J. Dong, X. Yu, C. Ning, L. Hu, N. Yu, Selective mono-arylation in palladiumcatalyzed cross-coupling reaction of dichlorotriazines with phenylboronate ester derivatives, Chin. Chem. Lett. 24 (2013) 41-44. |
| [23] | X. Wang, Y. Tian, Q. Zhang, J. Qi, D. Yin, An efficient synthesis of substituted 1,4-diazepines by a Pd catalyzed amination and sequential hydrogenation condensation, Chin. Chem. Lett. 24 (2013) 743-746. |
| [24] | L. Yu, P. Li, L. Wang, Copper-promoted decarboxylative direct C3-acylation of Nsubstituted indoles withα-oxocarboxylic acids, Chem. Commun. 49 (2013) 2368-2370. |
| [25] | R. Lang, L. Shi, D. Li, C. Xia, F. Li, A general method for palladium-catalyzed direct carbonylation of indole with alcohol and phenol, Org. Lett. 14 (2012) 4130-4133. |
| [26] | L. Joucla, L. Djakovitch, Transition metal-catalysed, direct and site-selective N1-C2-or C3-arylation of the indole nucleus: 20 years of improvements, Adv. Synth. Catal. 351 (2009) 673-714. |