Copper is an essential trace element and plays important physiological roles in many biological systems. But at the same time, copper is a significant metal pollutant due to its widespread use. Both deficiency and excess of copper can lead to health problems. Therefore the development of colorimetric or fluorescent sensors for the selective and sensitive detection of Cu2+ in biological and environmental systems has attracted considerable attention [1, 2, 3, 4, 5, 6, 7, 8, 9, 10].
Some metal ions can induce deprotonation of active NH, such as the NH groups conjugated to aromatic or carbonyl groups. This deprotonation process caused by complexation can be used formetal ions recognition and sensing. For the chemosensors based on intramolecular charge transfer, the deprotonations of coordination atoms in electron-donating groups can greatly enhance the electrondonating ability of electron donors and result in a redshift in emission and absorption spectra. Great achievement in the field of deprotonation- based chemosenors for metal ions has been made [6, 7, 8, 9, 10, 11, 12]. Guo reported two Zn2+ fluorescent sensors based onthe metal ion-induced deprotonation of an imide group, which show red-shifts of both excitation and emission bands due to an extensive π-system resulted from deprotonation of imide groups [11]. Inspired by the idea, we synthesized a new chemosensor (QDB) by coupling quinoline-2- carbaldehydewith 4-(dimethylamino)benzohydrazide. QDB exhibits a color change response from colorless to red only toward Cu2+.
Unless otherwise noted, all chemicals were obtained from commercial suppliers and used without further purification. 4-(Dimethylamino)benzohydrazide was prepared according to the procedure reported in the literature [13]. The corresponding metal nitrates were used as the metal cation sources. 1H NMR and 13C NMR spectra were recorded on a Bruker-400 spectrometer with TMS as the internal standard. ESI-MS spectra were performed on a Bruker esquire HCT-Agilent 1200 spectrometer. UV-vis absorption spectra were recorded on TU-1901 spectrophotometer. X-ray diffraction data collection was performed on Gemini A Ultra diffractometer.
Quinoline-2-carbaldehyde (0.30 g, 1.91 mmol) and 4-(dimethylamino) benzohydrazide (0.35 g, 1.95 mmol) were stirred at 72 ℃ in methanol (30 mL) under nitrogen atmosphere until complete consumption of quinoline-2-carbaldehyde, monitored by TLC (about 10 h). The reaction mixture was cooled to room temperature to give a precipitate. The precipitate was collected by filtration and washed three times with cooled methanol to afford QDB as a pale yellow solid (0.38 g, 63%). 1H NMR (DMSO-d6): δ 11.92 (s, 1H), 8.60 (s, 1H), 8.42 (d, 1H, J = 8.8 Hz), 8.12 (d, 1H, J = 8.8 Hz), 8.05 (d, 1H, J = 8.4 Hz), 8.02 (d, 1H, J = 8.4 Hz), 7.88 (d, 2H, J = 8.8 Hz), 7.77- 7.83 (m, 1H), 7.61-7.67 (m, 1H), 6.80 (d, 2H, J = 8.8 Hz), 3.03 (s, 6H). 13C NMR (DMSO-d6): δ 163.2, 154.2, 152.7, 147.4, 146.2, 136.6, 130.0, 129.5, 128.8, 128.0, 127.8, 127.1, 119.1, 117.4, 110.8, 39.7; ESI-MS m/z: 319.4 [M+H]+, 341.4 [M+Na]+.
Needle-like crystals suitable for X-ray analysis were obtained by evaporating a solution of QDB/Cu(NO3)2 (1:1 molar ratio) in ethanol.
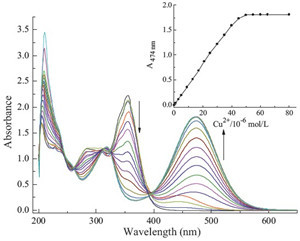
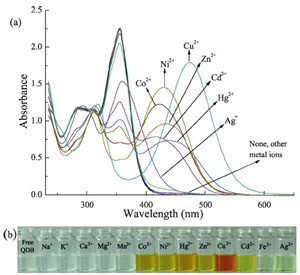
Free QDB shows absorption maximum at 356 nm in ethanol. Upon the gradual addition of Cu2+, the intensity of the absorption at 356 nm decreases and a new absorption band at 474 nm appears (Fig. 1), resulting in a color change from colorless to red. Other transition metal cations, such as Zn2+, Co2+ and Ni2+, also cause a decline of the absorbance at 356 nm and induce new absorption bands at longer wavelength than 356 nm. Nevertheless the induced new absorptions are lower in intensity and shorter at wavelength than that induced by Cu2+ (Fig. 2a), and Cu2+ is the only cation that causes an observable color change from colorless to red (Fig. 2b).
|
Download:
|
| Fig. 1.UV/vis spectra of QDB (5.0 × 10-5 mol/L) upon the titration of Cu2+ (0–1.6 equiv.) in ethanol. Inset: Absorbance at 474 nm as a function of Cu2+ concentrations. | |
|
Download:
|
| Fig. 2.(a) UV/vis responses of QDB (5.0 × 10-5 mol/L) to various metal ions in ethanol. (b) The color changes of QDB (5.0 × 10-5 mol/L) upon addition of various metal ions in ethanol. (Amount of the metal ions: 10 equiv. for Na+, K+, Ca2+, Mg2+ and 1.0 equiv. for other metal ions). | |
|
Download:
|
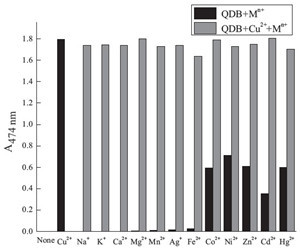
| Fig. 3.Absorbance of QDB in the presence of various cations (10 equiv. for Na+, K+,Ca2+, Mg2+ and 1.0 equiv. for other metal ions) at 474 nm (black bars) and the selective responses of QDB to Cu2+ (1.0 equiv.) in the presence of other cations ((10 equiv. for Na+, K+, Ca2+, Mg2+ and 1.0 equiv. for other metal ions) at 474 nm (gray bars) in ethanol. | |

|
Download:
|
| Fig. 4.(a) UV/vis responses of QDB (5.0 × 10-5 mol/L) to various metal cations (10 equiv. for Na+, K+, Ca2+,Mg2+ and 1.0 equiv. for other metal ions) in aqueous ethanol (50%, v/v). (b) Absorption responses of QDB in the presence of various metal cations (10 equiv. for Na+, K+, Ca2+, Mg2+ and 1.0 equiv. for other metal ions) (black bars) and in the presence of the mixtures of Cu2+ (1.0 equiv.) and various metal cations (10 equiv. for Na+, K+, Ca2+, Mg2+ and 1.0 equiv. for other metal ions) (gray bars) in aqueous ethanol (50% v/v). (c) The color changes of QDB (2.0 × 10-5 mol/L) in the presence of Cu2+ (1.0 equiv.), Ni2+ (1.0 equiv.) or their mixture (1.0 equiv. for each ion). | |
|
Download:
|
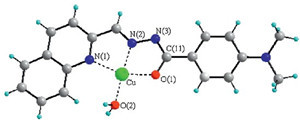
| Fig. 5.Coordination mode of QDB with Cu2+. The non-coordinated H2O and (NO3)- are omitted for clarity. Selected bond distances (Å): Cu–N(1) 2.079, Cu–N(2) 1.920,Cu–O(1) 1.981, Cu–O(2) 1.932, C(11)–O(1) 1.284, C(11)–N(3) 1.341. | |
In summary, we have developed a quinine-based colorimetric chemosensor for Cu2+ detection. The sensor displays an apparent response to Cu2+ by a significantly red-shifted absorption band and a color change fromcolorless to red. Othermetal ions have noeffect on its specific response to Cu2+. The 1:1 binding mode was proposed basedonUV-vis titrations, ESI-MSandX-ray analysis.When binding with Cu2+, the deprotonation process of NH in the sensor would occur, which causes the large redshift and the color change.
This work is supported by the National Natural Science Foundation of China (No. 21162010) and the College Students’ Innovation Training Project of Hainan Normal University (No. cxcyxj2013005).
| [1] | Y. Jeong, J. Yoon, Recent progress on fluorescent chemosensors for metal ions, Inorg. Chim. Acta 381 (2012) 2-14. |
| [2] | X. Chen, J. Wang, J. Cui, Z. Xu, X. Peng, A ratiometric and exclusively selective CuⅡ fluorescent probe based on internal charge transfer (ICT), Tetrahedron 67 (2011) 4869-4873. |
| [3] | Z. Xu, Y. Xiao, X. Qian, J. Cui, D. Cui, Ratiometric and selective fluorescent sensor for CuⅡ based on internal charge transfer (ICT), Org. Lett. 7 (2005) 889-892. |
| [4] | Y. Shiraishi, K. Tanaka, T. Hirai, Colorimetric sensing of Cu(Ⅱ) in aqueous media with a spiropyran derivative via a oxidative dehydrogenation mechanism, ACS Appl. Mater. Interfaces 5 (2013) 3456-3463. |
| [5] | C.D. Sun, J.M. Chen, H.M. Ma, et al., A new Cu2+-induced color reaction of a rhodamine derivative N-(3-carboxy)acryloyl rhodamine B hydrazide, Sci. China Chem. 54 (2011) 1101-1108. |
| [6] | J.H. Huang, Y.F. Xu, X.H. Qian, A colorimetric sensor for Cu2+ in aqueous solution based on metal ion-induced deprotonation: deprotonation/protonation mediated by Cu2+-ligand interactions, Dalton Trans. (2009) 1761-1766. |
| [7] | S.P. Wu, R.Y. Huang, K.J. Du, Colorimetric sensing of Cu(Ⅱ) by 2-methyl-3-[(pyridin-2-ylmethyl)-amino]-1,4-naphthoquinone: Cu(Ⅱ) induced deprotonation of NH responsible for color changes, Dalton Trans. (2009) 4735-4740. |
| [8] | Z.C. Xu, X.H. Qian, J.N. Cui, Colorimetric and ratiometric fluorescent chemosensor with a large red-shift in emission: Cu(Ⅱ)-only sensing by deprotonation of secondary amines as receptor conjugated to naphthalimide fluorophore, Org. Lett. 7 (2005) 3029-3032. |
| [9] | J. Jiang, H. Jiang, X. Tang, et al., An efficient sensor for Zn2+ and Cu2+ based on different binding modes, Dalton Trans. 40 (2011) 6367-6370. |
| [10] | Z. Xu, J. Pan, D.R. Spring, J. Cui, J. Yoon, Ratiometric fluorescent and colorimetric sensors for Cu2+ based on 4,5-disubstituted-1,8-naphthalimide and sensing cyanide via Cu2+ displacement approach, Tetrahedron 66 (2010) 1678-1683. |
| [11] | D.Y. Wu, L.X. Xie, C.L. Zhang, et al., Quinoline-based molecular clips for selective fluorescent detection of Zn2+, Dalton Trans. (2006) 3528-3533. |
| [12] | N. Zhang, Y. Su, M. Yu, A novel cell-impermeable fluorescent zinc sensor containing poly(ethylene glycol) chain, Chin. Chem. Lett. 22 (2011) 863-866. |
| [13] | H. Mu, R. Gong, L. Ren, et al., An intramolecular charge transfer fluorescent probe: Synthesis and selective fluorescent sensing of Ag+, Spectrochim. Acta A 70 (2008) 923-928. |




