Anion recognition is an area of growing interest in supramolecular chemistry due to its important role in a wide range of environmental, clinical, chemical, and biological applications [1, 2, 3, 4]. Cyanide detection is particularly important due to its extreme toxicity in physiological systems and the increasing environmental concerns caused by its widespread industrial uses in petrochemical, gold mining, photographic, and steel manufacturing [5, 6, 7, 8, 9]. To detect cyanide anions, fluorescence sensing is one of the most powerful methods owing to its simplicity and high sensitivity [10, 11, 12, 13].
In recent years, some organic molecules and transition metal complexes capable of signaling the presence of cyanide by pronounced changes in their absorption and emission properties have been already identified [14, 15, 16]. Some of these chemosensors can even detect micromolar amounts of cyanide [17, 18, 19]. However, most of them suffer the deleterious interference of other anions and in addition, many of them are reported to work only in organic media [20, 21, 22]. Consequently, the search for effective sensing systems in aqueous environment is still a great challenge.
With the above-mentioned criteria in mind, herein, we have designed 2,2'-bisbenzimidazole (L), which behaves as a highly sensitive fluorescence turn-on sensor for the cyanide anion in aqueous solutions. In this article, we illustrate the logic behind our molecular design and we report the synthesis and characterization of L with its fluorescent response to cyanide.
Melting points were measured on X-4 digital melting-point apparatus and were uncorrected. The infrared spectra were performed on a Digilab FTS-3000 FT-IR spectrophotometer. Mass spectra were measured with a Bruker Daltonics Esquire 6000. Fluorescence spectra were recorded on a Shimadzu RF-5301 fluorescence spectrometer. 1H NMR spectra were recorded on a Varian Mercury plus-400 MHz spectrometer with DMSO as solvent and analytical grade TMS as an internal reference.
All reagents were obtained commercially for synthesis and used without further purification. In the titration experiments, stock solutions of the tetrabutyl ammonium salts of F-, Cl-, Br-, I-, AcO-, H2PO4 -, HSO4 -, ClO4 -, but CN- was prepared in NaCN. All were purchased from Alfa-Aesar Chemical, stored in a vacuum desiccator containing self-indicating silica and dried fully before using.
The synthesis route of receptor molecule L is demonstrated in Scheme 1. Components O-phenylenediamine (5 mmol) and oxalic acid (2 mmol) were mixed in glycol (40 mL), with PPA (polyphosphate phosphoric acid) as a catalyst. Then, the resulting solution was stirred under refluxed conditions for 2 h at 160 ℃. After cooling to room temperature, the bright green precipitate was filtrated, washed with distilled water three times, then recrystallized with glacial acetic acid to get green crystals of L in 96% yield (mp > 300 ℃), 1H NMR (400 MHz, DMSO-d6): δ 11.91 (s, 2H), 7.77 (s, 1H), 7.57 (s, 1H), 7.30 (s, 2H), 7.07-7.14 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 115.263, 123.127, 143.923, 155.354, 172.210. IR (KBr, cm-1): v 1620.13 (CH55N), 3250.78 (NH). MS/ (EI): m/z 235.2 (L+H)+; Anal. Calcd. for C14H10N4: C 71.78, H 4.30, N 23.92; found: C 71.69, H 4.10, N 24.21.

|
Download:
|
| Scheme 1.Synthetic procedures for receptor L. | |
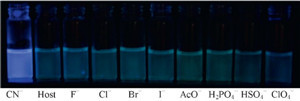
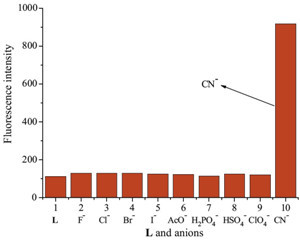
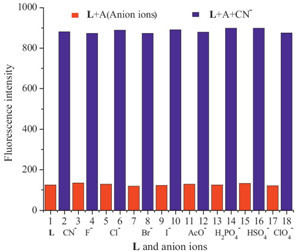
To evaluate the selectivity of L, we measured the fluorescence intensity of L in the presence of various anions (F-, CI-, Br-, I-, AcO-, H2PO4 -, HSO4 - and CIO4 -) as well as CN-. Only when 20 equiv. of CN- were added to the H2O/DMSO (1/1, v/v) solutions of sensor L, the chemosensor responded with a dramatic color change, from colorless to bright blue (Fig. 1). Receptor L produced a band at λmax = 365 nm in the absorption spectrum recorded at a 2 × 10-5 mol/L concentration of the receptor in a H2O system. Using these anions (20 equiv.), compound L showed a large fluorescent enhancement only with CN-. However, under identical conditions, no obvious changes were observed for other tested metal ions (Fig. 2).
|
Download:
|
| Fig. 1.Visual fluorescence emissions of probe L upon the addition of various anions (20 equiv.) in DMSO/H2O (1/1, v/v) solutions on excitation at 365 nm using UV lamp at r.t. | |
|
Download:
|
| Fig. 2.Fluorescence spectra of L and in the presence of 20 equiv. of various anions in H2O/DMSO (1/1, v/v) binary solution at room temperature. | |
|
Download:
|
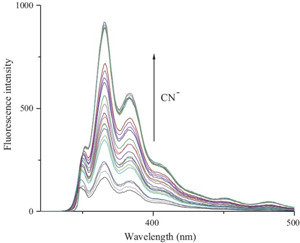
| Fig. 3.Fluorescence spectral titration of sensor L with CN- in (H2O/DMSO, 1/1, v/v) solution. | |
|
Download:
|
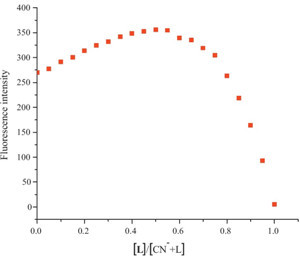
| Fig. 4.Job’s plot of L and CN-, which indicated the stoichiometry of L–CN- complex is 1:1. | |
|
Download:
|
| Fig. 5.Competitive experiments toward the fluorescence enhancement effect of CN- in presence of a large number of other anions (20 equiv.). | |
|
Download:
|
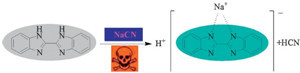
| Scheme 2.Possible sensing mechanism. | |
|
Download:
|
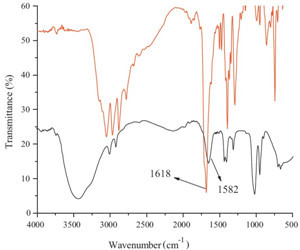
| Fig. 6.Infrared spectra of L (red line) and its complex (black line). | |
|
Download:
|
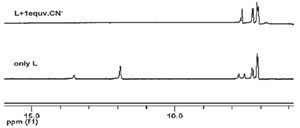
| Fig. 7.1H NMR spectra of receptor L (0.01 mol/L) upon addition of 1 equiv. of CN- in distilled water. | |
|
Download:
|
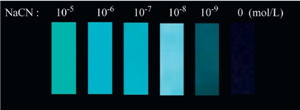
| Fig. 8.Detection limit in fluorescent mode. | |
|
Download:
|
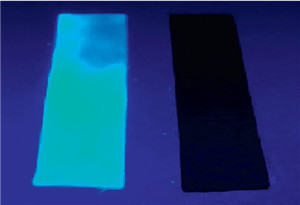
| Fig. 9.Fluorescence changes of test strips for detecting NaCN in aqueous solution using UV lamp at r.t. | |
A new simple and easily prepared 2,2'-bisbenzimidazole (L) described here displays fluorescence response driven by CN-. It can be synthesized easily and rapidly with good selectivity and sensitivity for CN-. As a result of the markable increases of the fluorescence intensity in the presence of CN- in H2O systems, it is possible for this ligand to directly sense and detect CN- by fluorescence intensity. These characteristics of L make it attractive for further molecular modifications and potential applications as fluorescence sensor for CN-.
This work was supported by the National Natural Science Foundation of China (Nos. 21064006, 21262032 and 21161018), the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (No. IRT1177), the Natural Science Foundation of Gansu Province (No. 1010RJZA018), the Youth Foundation of Gansu Province (No. 2011GS04735) and NWNU-LKQN-11-32.
| [1] | W.T. Gong, S. Bao, F.R. Wang, J.W. Ye, et al., Two-mode selective sensing of H2PO4- controlled by intramolecular hydrogen bonding as the valve, Tetrahedron Lett. 52 (2011) 630-634. |
| [2] | P. Saluja, N. Kaur, N. Singh, D.O. Jang, Benzimidazole-based fluorescent sensors for Cr3+ and their resultant complexes for sensing H2PO4- and F-, Tetrahedron 68 (2012) 8551-8556. |
| [3] | W. Lu, H. Jiang, F.Y. Hua, L.M. Jiang, Z.Q. Shen, A novel chemosensor based on Fe(Ⅲ)-complexation for selective recognition and rapid detection of fluoride anions in aqueous media, Tetrahedron 67 (2011) 7909-7912. |
| [4] | H. Tavallali, G. Deilamy-Rad, M. Tabandeh, A selective detection of fluoride ions in DMSO by fluorescent and colorimetry competition assays based on 4-bromo-2, Chin. Chem. Lett. 22 (2011) 193-196. |
| [5] | L. Deng, C.G. Chen, M. Zhou, S.J. Guo, et al., Integrated self-powered microchip biosensor for endogenous biological cyanide, Anal. Chem. 82 (2010) 4283-4287. |
| [6] | Y. Shiraishi, S. Sumiya, K. Manabe, T. Hirai, Thermoresponsive copolymer containing a coumarin_spiropyran conjugate: reusable fluorescent sensor for cyanide anion detection in water, Appl. Mater. Interfaces 3 (2011) 4649-4656. |
| [7] | H. Deng, Y.C. Qiu, C. Daiguebonne, et al., Synthesis of new copper cyanide complexes via the transformation of organonitrile to inorganic cyanide, Inorg. Chem. 47 (2008) 5866-5872. |
| [8] | J.L. Liu, Y. Liu, Q. Liu, C.Y. Li, et al., Iridium(Ⅲ) complex-coated nanosystem for ratiometric upconversion luminescence bioimaging of cyanide anions, J. Am. Chem. Soc. 133 (2011) 15276-15279. |
| [9] | Y.M. Dong, Y. Peng, M. Dong, Y.W. Wang, A selective, sensitive, and chromogenic chemodosimeter for cyanide based on the 1,10-binaphthyl scaffold, J. Org. Chem. 76 (2011) 6962-6966. |
| [10] | J. Yoshino, N. Kano, T. Kawashima, Fluorescence properties of simple N-substituted aldimines with a B-N interaction and their fluorescence quenching by a cyanide ion, J. Org. Chem. 74 (2009) 7496-7503. |
| [11] | Y.Y. Guo, X.L. Tang, F.P. Hou, et al., A reversible fluorescent chemosensor for cyanide in 100% aqueous solution, Sens. Actuators B 181 (2013) 202-208. |
| [12] | Y. Sun, S.W. Fan, L. Duan, R.F. Li, A ratiometric fluorescent probe based on benzo[e]indolium for cyanide ion in water, Sens. Actuators B 185 (2013) 638-643. |
| [13] | G.L. Fu, C.H. Zhao, An aggregation-induced emissive chromophore as a ratiometric fluorescent sensor for cyanide in aqueous media, Tetrahedron 69 (2013) 1700-1704. |
| [14] | L.J. Tang, P. Zhou, K.L. Zhong, S.H. Hou, Fluorescence relay enhancement sequential recognition of Cu2+ and CN- by a new quinazoline derivative, Sens. Actuators B 182 (2013) 439-445. |
| [15] | M. Shahid, S.S. Razi, P. Srivastava, et al., A useful scaffold based on acenaphthene exhibiting Cu2+induced excimer fluorescence and sensing cyanide via Cu2+ displacement approach, Tetrahedron 68 (2012) 9076-9084. |
| [16] | G. Absalan, M. Asadi, S. Kamran, S. Torabi, L. Sheikhian, Design of a cyanide ion optode based on immobilization of a new Co(Ⅲ) Schiff base complex on triacetylcellulose membrane using room temperature ionic liquids as modifiers, Sens. Actuators B 147 (2010) 31-36. |
| [17] | H.D. Li, B. Li, L.Y. Jin, Y.H. Kan, B.Z. Yin, A rapid responsive and highly selective probe for cyanide in the aqueous environment, Tetrahedron 67 (2011) 7348-7353. |
| [18] | Y.K. Yang, J. Tae, Acridinium salt based fluorescent and colorimetric chemosensor for the detection of cyanide in water, Org. Lett. 8 (2006) 5721-5723. |
| [19] | Y. Kim, H.S. Huh, M.H. Lee, et al., Turn-on fluorescence sensing of cyanide ions in aqueous solution at parts-per-billion concentrations, Chem. Eur. J. 17 (2011) 2057-2062. |
| [20] | G.J. Kim, H.J. Kim, Doubly activated coumarin as a colorimetric and fluorescent chemodosimeter for cyanide, Tetrahedron Lett. 51 (2010) 185-187. |
| [21] | A.O. El-Ballouli, Y.D. Zhang, S. Barlow, et al., Fluorescent detection of anions by dibenzophenazine-based sensors, Tetrahedron Lett. 53 (2012) 661-665. |
| [22] | M. Kumar, R. Kumar, V. Bhalla, Differential fluorogenic sensing of F- versus CN- based on thiacalix arene derivatives, Tetrahedron Lett. 54 (2013) 1524-1527. |




