b The State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, China
Materials with high birefringence have attracted great interest for their versatile application in technologies such as organic lightemitting diodes (OLEDs) [1], photostorage devices [2], optical fibers [3] and telecommunication devices [4]. Different materials are designed and synthesized to gain high birefringence including tolane [5, 6], dipheny-diacetylene [7], and azobenzene [8].
Among these materials, azobenzene and its derivatives are actively investigated [9]. Photoinduced anisotropy (PIA) and birefringence (PIB) were attributed to the repeated trans-cis-trans photoisomerization cycles of azobenzene chromophores, which endowed the materials containing azobenezene chromophores with various applications in optical information storage [10], polarization holographic gratings [11, 12], photomechanics [13], and so forth.
Ionic self-assembly (ISA) has emerged as an effective strategy for the design of novel functional materials [14, 15, 16]. This approach does not need rigorous and complicated synthetic processes compared to all-covalent polymers. In previous reports, ionic complexes containing azobenzene were prepared and showed photosensitivity under light irradiation. However, the birefringence was low and far from the requirement for practical application [17, 18, 19]. Therefore, design and synthesis of materials with high photoinduced orientation and inherent high birefringence by this facile method is still necessary.
Herein, we prepared a novel material (PANDAZO) with high birefringence by ISA of sodium polyacrylate (PANa) and azobenzene chromophores (NDAZO). The ionic complex shows lamellar structure. Under linear pulsed laser irradiation, the complex film exhibits high optical anisotropy. A high photoinduced birefringence up to 0.365 is achieved by using a continuous 488 nm linearly polarized laser as the pump light.
Sodium polyacrylate (PANa) with a molar mass (Mw) of 5100 was purchased from Aldrich. Sodium nitrite, phenol, N,Ndimethylaniline, N-methylimidazole, potassium carbonate, and the organic solvents utilized in this work were purchased from Sinopharm Chemical Reagent Co. and used without purification. 4- Hydroxy-4'-dimethylaminoazobenzene (1) was prepared according to the reported method [20]. FT-IR spectra were recorded on a Perkin-Elmer Paragon 1000 FTIR spectrometer. Normal and polarized absorption spectra of the complex films were recorded on a Perkin-Elmer lambda 750 UV/vis spectrometer. 1H NMR spectra were acquired on a Varian Mercury Plus 400 MHz spectrometer in DMSO-d6 or CDCl3 and the chemical shifts were referenced in ppm versus tetramethylsilane. Small-angle X-ray diffractogram (SXRD) patterns of the casting filmswere recorded on a Rigaku X-ray diffractometer D/MAX-2200/PC at a rate of 1°/min over the 2θ range 1-10°. Na+ and Br- analysis was performed using an FEI NanoSEM 230 scanning electron microscope equipped with an X-Max 80 energy-dispersive spectrometer (EDS).
4-Hydroxy-4'-dimethylaminoazobenzene 1 (2.41 g, 10 mmol) was dissolved in acetone, and then 2 equivalent 1,6-dibromohexane (4.83 g, 20 mmol) and 1.5 equivalent potassium carbonate (2.1 g, 15 mmol) were added to the solution. The mixture was refluxed for 24 h with stirring. The residue was filtered off and washed with ethyl acetate. The organic solvent was removed from the combined filtrate and washings under reduced pressure, and petroleum ether (60-90 ℃) was added to the concentrate. The resulting precipitate was collected and dried. The crude product was purified by recrystallization from ethanol. Yield: 3.1 g, 77%. 1H-NMR (400 MHz, CDCl3): δ 7.84 (4H, ArH), 6.97 (2H, ArH), 6.76 (2H, ArH), 4.02 (2H, OCH2), 3.43 (2H, BrCH2), 3.07 (6H, N(CH3)2), 1.91 (2H, CH2), 1.83 (2H, CH2), 1.52 (4H, CH2CH2).
Compound 2 (1.0 g, 2.48 mmol) was dissolved in 30 mL tetrahydrofuran. Then 1-methylimidazole (1.63 g, 19.84 mmol) was added to the solution. The mixture was heated to reflux for 72 h. The resulting precipitate was obtained by filtration and washed with tetrahydrofuran and diethyl ether, respectively. The filter cake was dried under vacuum to give NDAZO. Yield: 0.78 g, 65%. 1HNMR(400 MHz, DMSO-d6): δ 9.13 (1H,N55CH-N), 7.72 (6H, ArH, N-CH55CH-N), 7.03 (2H, ArH), 6.80 (2H, ArH), 4.17 (2H, OCH2), 4.02 (2H, NCH2), 3.84 (3H, NCH3), 3.02 (6H, N(CH3)2), 1.76 (4H, CH2CH2), 1.45 (2H, CH2), 1.30 (2H, CH2).
Aqueous PANa solution (5 mg/mL) was added dropwise to aqueous NDAZO solution (1 mg/mL) with a 1:1 molar charge ratio of cations to anions. The resulting precipitate was collected by filtration and washed thoroughly with deionized water to remove residual salts and noncomplexed precursors, and then dried in vacuum at 60 ℃ for 24 h. PANDAZO films were prepared by spincoating a chloroform/ethanol (9/1, v/v) solution (concentration: 20 mg/mL) onto quartz plates and glass slides (speed: 2000 rpm; time: 20 s) after being filtered through a 0.45 μm Millipore filter. The thickness of the resultant film was about 200-300 nm, as measured by means of Ellipsometer.
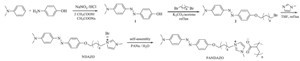
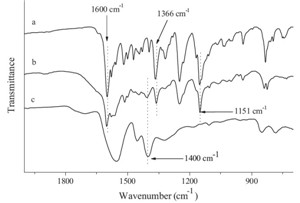
NDAZO unit was prepared according to Scheme 1. NDAZO unit showed good solubility in water. Ionic complex PANDAZO was obtained as a precipitate by adding PANa to the NDAZO solutions in a 1:1 charge ratio. PANDAZO showed excellent solubility in chloroform/ethanol (9/1, v/v), DMSO, and N-methy l-2-pyrrolidone (NMP). Fig. 1 shows the FT-IR spectra of NDAZO, PANDAZO, and PANa. The stretching vibration bands of the azo moieties in the complex were found at ca. 1600, 1366, and 1151 cm-1. The symmetric carboxylate stretch of the complex was found at 1400 cm-1. This result indicated that the NDAZO unit was successfully attached to the PANa main chain.
|
Download:
|
| Scheme 1.The synthetic routes to the NDAZO and PANDAZO. | |
|
Download:
|
| Fig. 1.FT-IR spectra of (a) NDAZO, (b) PANDAZO and (c) PANa. | |
|
Download:
|
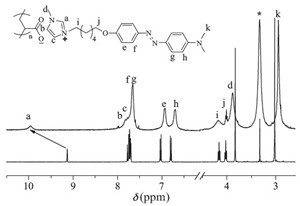
| Fig. 2.1H NMR spectra of PANDAZO (up) and NDAZO (down) in DMSO-d6. | |
|
Download:
|
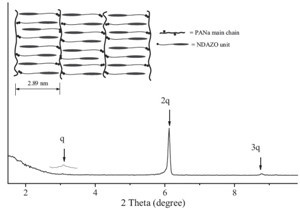
| Fig. 3.SXRD profile of the complex PANDAZO at room temperature. The inset is schematic representation of the layered architectures of the PANDAZO complex. | |
|
Download:
|
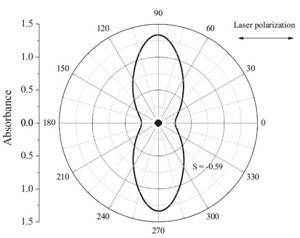
| Fig. 4.Angular-dependent polarized UV/vis spectra of PANDAZO at 395 nm after irradiation with 25 mW/cm2 by pulse polarization laser (355 nm). | |
|
Download:
|
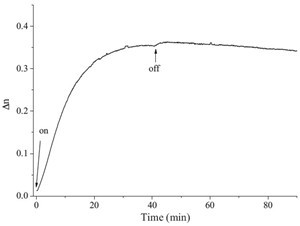
| Fig. 5.Photoinduced birefringence recorded in a thin film of PANDAZO with a 488 nm linearly polarized beam (165 mW/cm2) at room temperature. The points at which the pumping light was switched on and off are marked with arrows. | |
We prepared a photoresponding complex by ionic selfassembly. The ionic complex PANDAZO formed interdigitated lamellar microstructures with full overlap of the side chains. Effective photoinduced anisotropy (S = -0.59) was observed under irradiation by a pulsed laser. Polarized UV spectra revealed that the preferred direction of the azobenzene chromophores was perpendicular to the laser polarization direction. A high birefringence (Δn = 0.365) was induced by using a 488 nm linearly polarized laser as the pump light.
The authors gratefully acknowledge financial support from the National Science Fund for Distinguished Young Scholars (No. 50925310), the National Science Foundation of China (Nos. 50902094 and 51173103), the 973 Project (Nos. 2009CB93043 and 2012CB933803), and the Excellent Academic Leaders of Shanghai (No. 11XD1403000).
| [1] | M. O'Neill, S.M. Kelly, Liquid crystals for charge transport, luminescence, and photonics, Adv. Mater. 15 (2003) 1135-1146. |
| [2] | V. Shibaev, A. Bobrovsky, N. Boiko, Photoactive liquid crystalline polymer systems with light-controllable structure and optical properties, Prog. Polym. Sci. 28 (2003) 729-836. |
| [3] | T.R. Woliński, A. Szymańska, T. Nasiłowski, et al., Propagation effects in liquid crystal-core optical fiber waveguides, Mol. Cryst. Liq. Cryst. Sci. Technol. A: Mol. Cryst. Liq. Cryst. 321 (1998) 113-124. |
| [4] | K. Okano, A. Shishido, T. Ikeda, Photochemical phase transition behavior of highly birefringent azotolane liquid-crystalline polymer films: effects of the position of the tolane group and the donor-acceptor substituent in the mesogen, Macromolecules 39 (2005) 145-152. |
| [5] | Q. Song, S. Gauza, H. Xianyu, et al., High birefringence lateral difluoro phenyl tolane liquid crystals, Liq. Cryst. 37 (2010) 139-147. |
| [6] | C. Sekine, K. Iwakura, N. Konya, M. Minai, K. Fujisawa, Synthesis and properties of some novel high birefringence phenylacetylene liquid crystal materials with lateral substituents, Liq. Cryst. 28 (2001) 1375-1387. |
| [7] | S.T. Wu, U. Finkenzeller, V. Reiffenrath, Physical properties of diphenyldiacetylenic liquid crystals, J. Appl. Phys. 65 (1989) 4372-4376. |
| [8] | D. Wang, X. Wang, Amphiphilic azo polymers: molecular engineering, self-assembly and photoresponsive properties, Prog. Polym. Sci. 38 (2012) 271-301. |
| [9] | H.M.D. Bandara, S.C. Burdette, Photoisomerization in different classes of azobenzene, Chem. Soc. Rev. 41 (2012) 1809-1825. |
| [10] | S.K. Yesodha, C.K.S. Pillai, N. Tsutsumi, Stable polymeric materials for nonlinear optics: a review based on azobenzene systems, Prog. Polym. Sci. 29 (2004) 45-74. |
| [11] | A. Natansohn, P. Rochon, Photoinduced motions in azo-containing polymers, Chem. Rev. 102 (2002) 4139-4175. |
| [12] | A. Shishido, Rewritable holograms based on azobenzene-containing liquid-crystalline polymers, Polym. J. 42 (2010) 525-533. |
| [13] | T. Ikeda, J. Mamiya, Y.L. Yu, Photomechanics of liquid-crystalline elastomers and other polymers, Angew. Chem. Int. Ed. 46 (2007) 506-528. |
| [14] | T. Zhang, J. Brown, R.J. Oakley, C.F.J. Faul, Towards functional nanostructures: ionic self-assembly of polyoxometalates and surfactants, Curr. Opin. Colloid Interface Sci. 14 (2009) 62-70. |
| [15] | C.F.J. Faul, M. Antonietti, Ionic self-assembly: facile synthesis of supramolecular materials, Adv. Mater. 15 (2003) 673-683. |
| [16] | Z.Y. Cheng, B.Y. Ren, S.Y. He, X.X. Liu, Z. Tong, Mesomorphous structure change by tail chain number in ionic liquid crystalline complexes of linear polymer and amphiphiles, Chin. Chem. Lett. 22 (2011) 1375-1378. |
| [17] | S. Xiao, X. Lu, Q. Lu, Photosensitive polymer from ionic self-assembly of azobenzene dye and poly(ionic liquid) and its alignment characteristic toward liquid crystal molecules, Macromolecules 40 (2007) 7944-7950. |
| [18] | Q. Zhang, C.G. Bazuin, Liquid crystallinity and other properties in complexes of cationic azo-aontaining surfactomesogens with poly(styrenesulfonate), Macromolecules 42 (2009) 4775-4786. |
| [19] | M. Marcos, R. Alcala, J. Barbera, et al., Photosensitive ionic nematic liquid crystalline complexes based on dendrimers and hyperbranched polymers and a cyanoazobenzene carboxylic acid, Chem. Mater. 20 (2008) 5209-5217. |
| [20] | J.P.Hu, Y.F. Li, Z.Y. Ji, et al.,Anon-planar organicmoleculewithnon-volatile electrical bistability for nano-scale data storage, J. Mater. Chem. 17 (2007) 3530-3535. |
| [21] | X. Li, R. Wen, Y. Zhang, et al., Photoresponsive side-chain liquid crystalline polymers with an easily cross-linkable azobenzene mesogen, J. Mater. Chem. 19 (2009) 236-245. |
| [22] | P.Y. Vuillaume, C.G. Bazuin, Self-assembly of a tail-end pyridinium polyamphiphile complexed with n-alkyl sulfonates of variable chain length, Macromolecules 36 (2003) 6378-6388. |
| [23] | X. Sallenave, C.G. Bazuin, Interplay of ionic, hydrogen-bonding, and polar interactions in liquid crystalline complexes of a pyridylpyridinium polyamphiphile with (azo)phenol-functionalized molecules, Macromolecules 40 (2007) 5326-5336. |
| [24] | R.P. Nieuwhof, A.T.M. Marcelis, E.J.R. Sudholter, Thermotropic behavior of sidechain liquid-crystalline copolymers from maleic anhydride and mesogen-containing methacrylates, Macromol. Chem. Phys. 200 (1999) 2494-2500. |
| [25] | S.F. Xiao, X.M. Lu, Q.H. Lu, B. Su, Photosensitive liquid-crystalline supramolecules self-assembled from ionic liquid crystal and polyelectrolyte for laser-induced optical anisotropy, Macromolecules 41 (2008) 3884-3892. |




