3-Methylindole,one of the most important indole derivatives,is widely used in perfumes and the manufacture of herbicides, fungicides,dyes,antihypertensive and anticancer medicines,etc. [1, 2, 3, 4]. For many years,the synthesis of 3-methylindole has been an area of focus for organic chemists,and numerous synthesis methods have been developed [5, 6]. Among them,liquid phase synthesis can give 3-methylindole in a good yield,however,some drawbacks existed,such as the use of complicated and expensive reagents or catalysts,a large number of toxic solvents,heavy pollution and severe reaction conditions,etc. [7, 8, 9],which limit the further development of 3-methylindole. From economic and environmental viewpoints,therefore,vapor-phase synthesis of 3-methylindole is now more attractive. Subrahmanyam and coworkers investigated the vapor-phase synthesis of 3-methylindole from methanol and indole over Ce/HY catalyst,obtaining a 30% yield [10]. Campanati et al. reported the synthesis of 3- methylindole from 1,2-propanediol and aniline over ZrO2/SiO2 catalyst,but the yield of 3-methylindole was only 12% [11]. Recently,our group developed a new approach for the vapor-phase synthesis of 3-methylindole with glycerol and aniline as reactants (Scheme 1). Over Cu/SiO2-Al2O3,the yield of 3-methylindole reached 40% [12].
|
Download:
|
| scheme 1 Vapor-phase synthesis of 3-methylindole from glycerol and aniline. | |
Today,more and more attention focuses on the production of value-added chemicals from renewable resources such as biomass [13, 14, 15]. Glycerol,which comes from biomass,is not only cheap and abundant,but also known as a green renewable feedstock. Therefore,the vapor-phase synthesis of 3-methylindole from glycerol and aniline is a promising route.
Zeolites have attracted increasing interest in the manufacture of fine chemicals and chemical intermediates owing to their special features such as the acidity,inherent product selectivity, commercial availability,non-corrosive and eco-friendly nature, etc. [16, 17, 18]. In this paper,Cu/NaY and the catalyst modified by K2O were used for the vapor-phase synthesis of 3-methylindole from glycerol and aniline. XRD and NH3-TPD techniques were used to reveal the relationship between the structure of catalysts and their catalytic performances. The effect of reaction temperature was also investigated. In addition,a probable catalytic mechanism for the synthesis of 3-methylindole was proposed.
2. ExperimentalCu/NaY catalyst was prepared by incipient wetness impregnation. After impregnating NaY (20-40 mesh) with copper nitrate aqueous solution for 15 h at room temperature,the material was dried at 120 ℃ for 4 h,and then calcined at 500 ℃ for 4 h to obtain the catalyst precursor. Cu/NaY-K2O catalyst was prepared by sequential impregnation. First,NaY support was pre-impregnated with an aqueous solution of potassium acetate,then dried and calcined to get NaY-K2O. Afterwards,NaY-K2O was impregnated with an aqueous solution of copper,followed by drying and calcining to obtain the catalyst precursor of Cu/NaY-K2O,in which the procedure of impregnating,drying or calcining was the same as Cu/NaY described above. Prior to the activity test,3 mL catalyst precursor was in situ reduced in a mixture of gas composed of N2 (30 mL/min) and H2 (30 mL/min) at 250 ℃ for 2 h,then cooled to reaction temperature in 0.5 h and kept at the temperature for another 0.5 h. Copper loading was 0.9 mmol/g. The amount of K2O promoter was 0.4 mmol/g.
X-ray diffraction (XRD) patterns were recorded on a D8 Advance X-ray diffractometer with a Cu Kα radiation source at a scan rate of 0.5 °/min in the 2θ range of 30°-80°. The voltage and current were 40 kV and 40 mA,respectively. The temperatureprogrammed desorption of ammonia (NH3-TPD) was performed in a quartz reactor with inside diameter of 6 mm and length of 350 mm. 150 mg of the sample was pretreated at 500 ℃ for 1 h in a flow of ultrapure helium gas (35 mL/min) to remove water and other contaminants from the catalyst and cooled down to 100 ℃, then saturated with ammonia gas at 100 ℃. After the sample was purged with helium gas (35 mL/min) at 100 ℃ for 2 h to remove physisorbed ammonia,TPD was carried out from 100 ℃ to 700 ℃ with a temperature ramp of 10 ℃/min.
The catalytic reactions were carried out in a fix-bed continuous flow glass reactor with inside diameter of 12 mm under atmospheric pressure. Aniline and glycerol were mixed in a molar ratio of 3:1. The total space velocity (SV) and liquid hourly space velocity (LHSV) of raw materials were 1700 h-1 and 0.4 h-1, respectively. The mixture of reactants was pumped through the preheater where they were vaporized and then entered into the reactor with flowing H2 (10 mL/min),steam (12 mL/min) and N2 (58 mL/min). The products were analyzed on a gas chromatograph with a mass spectrometer (Shimadzu GCMS-QP2010) using a DB-5 capillary column and a gas chromatograph connected to a hydrogen flame ionization detector (FID-GC,SP-6800 A) using OV-17 column. The quantitative analysis of the reactants and products was carried out on the SP-6890A gas chromatograph equipped with an SE-54 capillary column. 1-Hexyl alcohol was used as an internal standard.
3. Results and discussionTable 1 shows the activity and selectivity of Cu/SiO2-Al2O3,Cu/ NaY or Cu/NaY-K2O. Compared with Cu/SiO2-Al2O3,Cu/NaY showed lower activity and selectivity. After adding K2O to Cu/ NaY,although the conversion of glycerol decreased,the selectivity of 3-methylindole increased remarkably,which resulted in the increase of 3-methylindole yield to 43%.
| Table 1 The activity and selectivity of Cu/SiO2-Al2O3,Cu/NaY or Cu/NaY-K2O. |
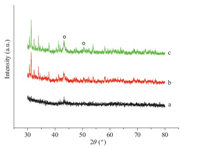
Fig. 1 illustrated the XRD patterns of Cu/SiO2-Al2O3,Cu/NaY and Cu/NaY-K2O. A small diffraction peak of copper at 2θ of 43.3°, assigned to the (1 1 1) reflection,was observed on Cu/SiO2-Al2O3 [19]. On NaY supported Cu-based catalysts,the intensity of copper diffraction peaks became stronger and another diffraction peak at 50.5°,assigned to the (2 0 0) reflection of copper,was observed. Moreover,the intensity of copper diffraction peaks on Cu/NaY-K2O was stronger than that on Cu/NaY,indicating that the addition of K2O to Cu/NaY was not conducive to the dispersion of copper particles on the support.
|
Download:
|
| Fig 1 XRD patterns of Cu/SiO2-Al2O3 (a),Cu/NaY (b) and Cu/NaY-K2O (c). | |
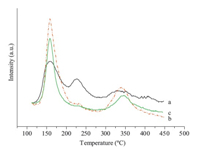
Fig. 2 showed NH3-TPD profiles of Cu/SiO2-Al2O3,Cu/NaY and Cu/NaY-K2O. More than two desorption peaks were observed. The peak at the lower temperature (<200 ℃) was assigned to the weak acid sites,while the others at the higher temperature (200-450 ℃) corresponded to the middle-strong acid sites [20]. The amount of the acid sites on the three catalysts decreased in the order of Cu/ SiO2-Al2O3,Cu/NaY and Cu/NaY-K2O. On Cu/SiO2-Al2O3,there were many weak acid sites and middle-strong acid sites. On Cu/NaY,the amount of weak acid sites or middle-strong acid sites that appeared at about 342 ℃ increased greatly,while the desorption peak corresponding to the middle-strong acid sites of 232 ℃ became very small. On Cu/NaY-K2O,only two desorption peaks corresponding to weak acid sites and middle-strong acid sites of 342 ℃ were observed,moreover,the amount of weak acid sites or middle-strong acid sites decreased significantly. Taking into account the results of NH3-TPD and Table 1,it can easily be inferred that the more the acid sites,the higher the conversion of glycerol,and a smaller amount of middle-strong acid sites was favorable to the higher selectivity of 3-methylindole.
|
Download:
|
|
Fig 2 NH3-TPD profiles of Cu/SiO2-Al2O3 (a),Cu/NaY (b) and Cu/NaY-K2O (c). |
|
For the vapor-phase synthesis of 3-methylindole from glycerol and aniline,it is known that acid sites can facilitate the adsorption of reactants on the surface of the catalyst,which is favorable to the conversion of glycerol [12]. Middle-strong acid sites,however, unfavorably obtain the high selectivity for the target product. Because 3-methylindole possesses weak alkalinity,it is difficult for 3-methylindole to desorb from the middle-strong acid sites,as a result,a further reaction of 3-methylindole can happen to form the by-products such as 1,3-dimethylindole,2,3-dimethylindole or 3- ketoneindole,etc. The characterizations of XRD and NH3-TPD show that K2O increased the selectivity of 3-methylindole due to its ability to decrease the amount of middle-strong acid sites significantly.
Table 2 shows the effect of reaction temperature on the activity and selectivity of Cu/NaY-K2O catalyst. It can be seen that high reaction temperature was detrimental to obtaining the high selectivity of 3-methylindole because glycerol could easily be converted to form by-products such as N-isopropylaniline,N-propylaniline, propylene glycol,indole,2,5-dimethyl-1-phenylpyrrole, N-isopropylindole and N,N’-dimethyl-N,N’-diphenyl-1,2- ethanediamine,etc. Considering the results of glycerol conversion and 3-methylindole selectivity,the optimal temperature for the reaction was 220 ℃,at which the selectivity of 3-methylindole was 75% and the yield of the target product reached 47%.
| Table 2 Effect of reaction temperature on the activity and selectivity of Cu/NaY-K2O catalyst. |
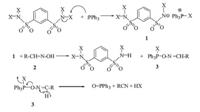
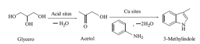
In order to know the mechanism of the synthesis of 3- methylindole from aniline and glycol,the reaction of glycerol with aniline and the conversion of glycerol over zeolites-supported Cubased catalysts were studied and acetol was found to be the major product for the conversion of glycerol. Based on the results of the catalytic reactions and the relevant literature [21, 22],a probable mechanism for the catalytic synthesis of 3-methylindole was hypothesized (Scheme 2). Firstly,glycerol was treated by dehydration to form an intermediate of acetol on acid sites,then the acetol reacted with aniline on Cu sites to form the target product of 3-methylindole after losing two molecules of water.
|
Download:
|
| scheme 2 A probable catalytic mechanism for the synthesis of 3-methylindole from glycerol and aniline. | |
The catalysts of Cu/NaY and Cu/NaY-K2O were used for the vapor-phase synthesis of 3-methylindole from glycerol and aniline. Although K2O did not promote the dispersion of copper particles on the support,it could decrease the amount of middlestrong acid sites,as a result,the selectivity of the catalyst increased significantly. In addition,the decrease of the reaction temperature was also favorable to the increase of 3-methylindole selectivity. Over Cu/NaY-K2O,when the temperature was 220 ℃,the selectivity of 3-methylindole reached 75% and the yield of the target product was up to 47%. A possible mechanism for the synthesis of 3-methylindole from glycerol and aniline over zeolites-supported Cu-based catalysts was proposed,in which acetol was the intermediate to produce 3-methylindole.
AcknowledgmentsWe gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (No. 21173110).
| [1] | M. Howe-Grant, Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed, John Wiley & Sons, New York, 1995, pp. 161-162. |
| [2] | M. Howe-Grant, Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed, John Wiley & Sons, New York, 1989, pp. 213-214. |
| [3] | B.B. Aggarwal, S. Shishodia, Molecular targets of dietary agents for prevention and therapy of cancer, Biochem. Pharm. 71 (2006) 1397-1421. |
| [4] | Z.A. Kaplanclkl, G. Turan-Zitouni, A. Özdemir, G. Revial, New triazole and triazolothiadiazine derivatives as possible antimicrobial agents, Eur. J. Med. Chem. 43 (2008) 155-159. |
| [5] | G.R. Humphrey, J.T. Kuethe, Practical methodologies for the synthesis of indoles, Chem. Rev. 106 (2006) 2875-2911. |
| [6] | M. Bandini, A. Eichholzer, Catalytic functionalization of indoles in a new dimension, Angew. Chem. Int. Ed. 48 (2009) 9608-9644. |
| [7] | C.A. Simoneau, A.M. Strohl, B. Ganem, One-pot synthesis of polysubstituted indoles from aliphatic nitro compounds under mild conditions, Tetrahedron Lett. 48 (2007) 1809-1811. |
| [8] | P. Magnus, I.S. Mitchell, Synthesis of 3-methylindoles from N-aryl-N-(3-triisopropylsilylpropargyl) sulfonamides, Tetrahedron Lett. 39 (1998) 4595-4598. |
| [9] | C.S. Cho, J.H. Kim, T.J. Kim, S.C. Shim, Ruthenium-catalyzed heteroannulation of anilines with alkanolammonium chlorides leading to indoles, Tetrahedron 57 (2001) 3321-3329. |
| [10] | D.V. Gopal, B. Srinivas, V. Durgakumari, M. Subrahmanyam, Vapor-phase alkylation of indole with methanol over zeolites, Appl. Catal. A 224 (2002) 121-128. |
| [11] | M. Campanati, S. Franceschini, O. Piccolo, A. Vaccari, Reaction pathway in the vapor-phase synthesis of indole and alkylindoles, J. Catal. 232 (2005) 1-9. |
| [12] | W. Sun, D.Y. Liu, H.Y. Zhu, Q. Sun, L. Shi, A new efficient approach to 3-methylindole: vapor-phase synthesis from aniline and glycerol over Cu-based catalyst, Catal. Commun. 12 (2010) 147-150. |
| [13] | A. Behr, J. Eilting, K. Irawadi, J. Leschinski, F. Lindner, Improved utilisation of renewable resources: new important derivatives of glycerol, Green Chem. 10 (2008) 13-30. |
| [14] | A. Corma, G.W. Huber, L. Sauvanaud, P. O'Connor, Biomass to chemicals: catalytic conversion of glycerol/water mixtures into acrolein, reaction network, J. Catal. 257 (2008) 163-171. |
| [15] | C.B. Lu, J.Z. Yao, W.G. Lin, W.L. Song, Study on biomass catalytic pyrolysis for production of bio-gasoline by on-line FTIR, Chin. Chem. Lett. 18 (2007) 445-448. |
| [16] | M. Karthik, C.J. Magesh, P.T. Preumal, et al., Zeolite-catalyzed ecofriendly synthesis of vibrindole A and bis(indolyl)methanes, Appl. Catal. A 286 (2005) 137-141. |
| [17] | M.A. Alotaibi, E.F. Kozhevnikova, I.V. Kozhevnikov, Hydrogenation of methyl isobutyl ketone over bifunctional Pt-zeolite catalyst, J. Catal. 293 (2012) 141- 144. |
| [18] | H.F. Shi, Y.C. Hu, Y. Wang, H. Huang, KNaY-zeolite catalyzed dehydration of methyl lactate, Chin. Chem. Lett. 18 (2007) 476-478. |
| [19] | S.Y. Zhang, Q.Y. Liu, G.L. Fan, Highly-dispersed copper-based catalysts from Cu- Zn-Al layered double hydroxide precursor for gas-phase hydrogenation of dimethyl oxalate to ethylene glycol, Catal. Lett. 142 (2012) 1121-1127. |
| [20] | D.F. Jin, Z.Y. Hou, L.W. Zhang, X.M. Zheng, Selective synthesis of para-para0- dimethyldiphenylmethane over H-beta zeolite, Catal. Today 131 (2008) 378-384. |
| [21] | R.B. Mane, C.V. Rode, Simultaneous glycerol dehydration and in situ hydrogenolysis over Cu-Al oxide under an inert atmosphere, Green Chem. 14 (2012) 2780- 2789. |
| [22] | J. Zhao, W.Q. Yu, C. Chen, et al., Ni/NaX: a bifunctional efficient catalyst for selective hydrogenolysis of glycerol, Catal. Lett. 134 (2010) 184-189. |