b Department of Chemistry, Faculty of Sciences, Ilam University, P. O. Box 69315516, Ilam, Iran
Nitriles are important precursors in organic synthesis,used as intermediates for the synthesis of esters,amides,carboxylic acids, amines,and nitrogen-containing heterocycles [1]. There are many reportedprocedures for the synthesis of nitriles,such as nucleophilic substitution of alkyl halides with metal cyanide for alkyl nitriles [2], and the Sandmeyer andammoxidation reaction for aromatic nitriles (benzonitriles) [3]. Dehydration of aldoximes to nitriles is one of the cleanest routes,avoiding inorganic cyanides. Many methods have been used for the dehydration of aldoximes into nitriles; such as Pd(OAc)2/PPh3 in CH3CN [4a],benzotriazole phosphonium hexafluorophosphate derivative/DBU in CH2Cl2 [4b],N-chlorosuccinimide/ pyridine in CH3CN [4c],DMF at 135 ℃ [4d],tungsten-tin mixed hydroxide in o-xylene at 149 ℃ [4e],diethylchlorophosphate in toluene [4f],molecular sieves under flash vacuum pyrolysis [4g], ZnO/CH3COCl [4h],chlorosulfonic acid in toluene [4i],dimethylthiocarbonate/ Et3N in dioxane [4j],diethylchlorophosphite in CHCl3 [4k],zeolite under microwave irradiation [4l],AlCl3·6H2O/KI/H2O/ CH3CN [4m],Preyssler’s anion,[NaP5W30O110] [4n],Burgess reagent [4o],thionyl chloride [4p],and PPh3/NCS [4q],N-chlorosuccinimide/ pyridine [4c],[pmim]BF4 [4r],trichloroisocyanuric acid [4s],Ntriflylimidazole [4t],triphenylphosphine oxide/oxalyl chloride [4u], MFR-H2SO4 [4v],as dehydrating agents. However,these procedures have some drawbacks,such as low yields,long reaction times, expensive reagents,heavymetal contaminations and harsh reaction conditions. Therefore,to improve the mentioned limitations,we decided to apply a new reaction media for the conversion of aldoximes to nitriles.
N,N,N',N'-Tetrabromobenzene-1,3-disulfonamide (TBBDA) and N,N,N',N'-tetrachlorobenzene-1,3-disulfonamide (TCBDA) are halogenating agents,and are effective catalysts and reagents for various organic transformations [5]. Since TCBDA and TBBDA contain bromine and chlorine atoms which are attached to the nitrogen atoms,it is very probable that they release in situ Br+ and Cl+,which can act as an electrophilic species,and interaction of Ph3P with TBBDA or TCBDA would generate phosphonium halides as the reactive phosphonium species.
2. Experimental 2.1. Typical procedure for the conversion of 4-nitrobenzaldehyde oxime to 4-nitrobenzonitrile with TCBDA and PPh3To a mixture of PPh3 (0.315 g,1.2 mmol) and TCBDA (0.12 g, 0.32 mmol) in dichloromethane (5 mL),4-nitrobenzaldehyde oxime (0.166 g,1 mmol) was added. The mixture was stirred at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction (Table 1),the solvent was evaporated. The crude products were purified by short-column chromatography (packed with silica gel,using n-hexane/ethyl acetate (8:2) as eluent) to achieve the desired 4-nitrobenzonitrile with 0.14 g,95% yield.
| Table 1 Dehydration of the aldoximes to the corresponding nitriles with TCBDA and TBBDA.a |
To the mixture of PPh3 (0.525 g,2 mmol) and TBBDA (0.287 g, 0.53 mmol) in dry acetonitrile (5 mL),4-nitrobenzaldehyde oxime (0.166 g,1 mmol) was added. The mixture was stirred at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction (Table 1),the solvent was evaporated. The crude products were purified by short-column chromatography (packed with silica gel,using n-hexane/ethyl acetate (8:2) as eluent) to achieve the desired 4-nitrobenzonitrile with 0.13 g,92% yield.
4-Nitrobenzonitrile (Table 1,entry 7): mp 143-147 ℃: IR (KBr, cm-1): 3106,2233,1602,1525,1349; 1H NMR (400 MHz,CDCl3): δ 8.39 (d,2H,J = 8.8 Hz),7.92 (d,2H,J = 8.8 Hz). 13C NMR (100 MHz, CDCl3): δ 150.06,133.50,124.32,118.36,116.82.
3-Nitrobenzonitrile (Table 1,entry 8): mp 114-117 ℃: IR (KBr, cm-1): 3110,2232,1609,1525,1344; 1H NMR (400 MHz,CDCl3): δ 8.58 (s,1H),8.52 (d,1H,J = 9.6 Hz),8.03 (d,1H,J = 8 Hz),7.78 (t,1H, J = 8 Hz). 13C NMR (CDCl3,100 MHz): δ 148.27,137.61,130.68, 127.56,127.27,116.55,114.18.
2-Nitrobenzonitrile (Table 1,entry 9): mp 105-109 ℃: IR (KBr, cm-1): 3112,2232,1613,1530,1344; 1H NMR (400 MHz,CDCl3): δ 8.39 (m,1H),7.96 (m,1H),7.88 (m,2H). 13C NMR (100 MHz, CDCl3): δ 148.68,135.63,134.31,133.70,125.60,114.95,108.17.
4-Methoxybenzonitrile (Table 1,entry 10): mp 57-59 ℃: IR (KBr,cm-1): 3086,2972,2224,1599; 1H NMR (400 MHz,CDCl3): δ 7.62 (d,2H,J = 8.8 Hz),6.98 (d,2H,J = 8.8 Hz),3.89 (s,3H). 13C NMR (100 MHz,CDCl3): δ 162.86,134.02,119.27,114.76,103.98,55.57.
2,6-Dichlorobenzonitrile (Table 1,entry 13): mp 142-144 ℃: IR (KBr,cm-1): 3092,2233,1572,802; 1H NMR (400 MHz,CDCl3): δ 7.53-7.45 (m,3H). 13C NMR (100 MHz,CDCl3): δ 138.55,133.86, 128.18,114.47,113.39.
2,4-Dichlorobenzonitrile (Table 1,entry 14): mp 56-58 ℃: IR (KBr,cm-1): 3066,2233,1581,821; 1H NMR (400 MHz,CDCl3): δ 7.65 (d,1H,J = 8.4 Hz),7.58 (d,1H,J = 2 Hz),7.41 (d,1H,J = 8.4 Hz). 13C NMR (100 MHz,CDCl3): δ 140.13,137.86,134.61,130.31, 127.88,115.27,111.91.
2-Nitrocinnamonitrile (Table 1,entry 18):mp87-90 ℃: IR (KBr, cm-1): 3075,2226,1605,1568,1523,1342; 1H NMR (400 MHz, CDCl3): δ 8.16 (d,1H,J = 9.2 Hz),8.0 (d,1H,J = 17.6 Hz),7.77-7.60 (m,3H),5.89 (d,1H,J = 16.4 Hz). 13C NMR (CDCl3,100 MHz): δ 147.48,146.68,134.09,131.36,129.68,128.75,125.36,116.96, 101.55.
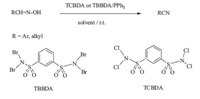
3. Results and discussionIn continuation of our interest in the application of TBBDA and TCBDA,in organic synthesis [5],herein we report a simple and improved protocol for the preparation of nitriles from aldoximes.The reactions proceeded via combination of TBBDA or TCBDA with Ph3P in solvent at room temperature under mild conditions (Scheme 1).
|
Download:
|
| scheme 1 Synthesis of nitriles from aldoximes. | |
In order to optimize the reaction conditions,we first examined the effect of different molar ratios of TBBDA/triphenylphosphine in CH3CN or TCBDA/triphenylphosphine in CH2Cl2 at room temperature for the conversion of 4-chlorobenzaldehye oxime to 4- chlorobenzonitrile as a model reaction. We found that the optimized molar ratio for transformation was 1/0.32/1.2 (4- chlorobenzaldehye oxime/TCBDA/PPh3) and 1/0.53/2 (4-chlorobenzaldehye oxime/TBBDA/PPh3). This method is general and can be easily applied for the conversion of aldoximes to their corresponding nitriles using the optimized molar ratios of TCBDA and/or TBBDA and Ph3P (Table 1).
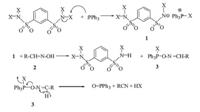
Dehydration reactions of aldoxime with TBBDA and TCBDA do not proceed in the absence of PPh3. Triphenylphosphine is a relatively general reducing agent,and its reactions with N-halo compounds can lead to the formation of phosphonium intermediates. The phosphorus has positive charge in these intermediates, so its reaction as a strong oxophilic reagent in most cases is driven to the formation of thermodynamically preferred triphenylphosphine oxide. Enthalpies of formation of triphenylphosphine and triphenylphosphine oxide are ΔfH0 = +207.02 kJ/mol and ΔfH0 = -116.41 kJ/mol,respectively [6]. As such,the formation of Ph3P=O is the major driving force in the proposed mechanism (Scheme 2).
|
Download:
|
| scheme 2 Proposed mechanism for the preparation of nitriles from aldoximes. | |
In this potential pathway,triphenylphosphine (PPh3) attacks N-halosufonamide compounds,and leads to intermediate 1 in which the phosphorus atom is more electrophilic. Then,nucleophilic attacks of aldoxime 2 on this intermediate give compound 3. Finally,intermediate 3 can be converted to the product via formation of triphenylphosphine oxide and HX.
4. ConclusionIn conclusion,TBBDA/Ph3P and TCBDA/Ph3P are mild and efficient reagent systems for the conversion of aldoximes into nitriles. Simple work-up,high yields,short reaction times and mild reaction temperature could also be considered advantages of this methodology.
AcknowledgmentsWe are thankful to Bu-Ali Sina University,Center of Excellence and Development of Chemical Methods (CEDCM) for financial support.
| [1] | (a) K. Friedrick, K. Wallensfels, The Chemistry of the Cyano Group, Wiley, New York, 1970p. 67;(b) M. North, A.R. Katritzky, O. Meth-Cohn, C.W. Rees, Comprehensive Organic Functional Group Transformations, Pergamon, Oxford, 1995;(c) S.I. Murahashi, Synthesis from nitriles with retention of the cyano group, Sci. Synth. Georg Thieme 19 (2004) 345-402;(d) S.J. Collier, P. Langer, Application of nitriles as reagents for organic synthesis with loss of the nitrile functionality, Sci. Synth. Georg Thieme 19 (2004) 403-425. |
| [2] | S. Patai, Z. Rappaport, A.J. Fatiadi, Preparation and Synthetic Applications of Cyano Compounds, Wiley, New York, 1983. |
| [3] | (a) G.P. Ellis, T.M. Romney-Alexande, Cyanation of aromatic halides, Chem. Rev. 87 (1987) 779-794;(b) R. Raja, R.D. Adams, D.A. Blom, et al., New catalytic liquid-phase ammoxidation approach to the preparation of niacin (vitamin B3), Langmuir 25 (2009) 7200-7204. |
| [4] | (a) H.S. Kim, S.H. Kim, J.N. Kim, Highly efficient Pd-catalyzed synthesis of nitriles from aldoximes, Tetrahedron Lett. 50 (2009) 1717-1719;(b) M.K. Singh, M.K. Lakshman, A simple synthesis of nitriles from aldoximes, J. Org. Chem. 74 (2009) 3079-3084;(c) M. Gucma, W.M. Golebiewski, Convenient conversion of aldoximes into nitriles with N-chlorosuccinimide and pyridine, Synthesis (2008) 1997-1999;(d) P. Supsana, T. Liaskopoulos, P.G. Tsoungas, G. Varvounis, DMF-catalysed thermal dehydration of aldoximes: a convenient access to functionalized aliphatic and aromatic nitriles, Synlett (2007) 2671-2674;(e) K. Yamaguchi, H. Fujiwara, Y. Ogasawara, M. Kotani, N. Mizuno, A tungsten-tin mixed hydroxide as an efficient heterogeneous catalyst for dehydration of aldoximes to nitriles, Angew. Chem. Int. Ed. 46 (2007) 3922-3925;(f) A.R. Sardarian, Z. Shahsavari-Fard, H.R. Shahsavari, Z. Ebrahimi, Efficient Beckmann rearrangement and dehydration of oximes via phosphonate intermediates, Tetrahedron Lett. 48 (2007) 2639-2643;(g) J.A. Campbell, G. McDougald, H. McNab, L.V.C. Rees, R.G. Tyas, Laboratory-scale synthesis of nitriles by catalysed dehydration of amides and oximes under flash vacuum pyrolysis (FVP) conditions, Synthesis (2007) 3179-3184;(h) M.H. Sarvari, ZnO/CH3COCl: a new and highly efficient catalyst for dehydration of aldoximes into nitriles under solvent-free condition, Synthesis (2005) 787-790;(i) D. Li, F. Shi, S. Guo, Y. Deng, Highly efficient Beckmann rearrangement and dehydration of oximes, Tetrahedron Lett. 46 (2005) 671-674;(j) T.A. Khan, S. Peruncheralathan, H. Ila, H. Junjappa, S,S-Dimethyl dithiocarbonate: a useful reagent for efficient conversion of aldoximes to nitriles, Synlett (2004) 2019-2021;(k) Z. Jie, V. Rammoorty, B. Fischer, Diethyl chlorophosphite: a versatile reagent, J. Org. Chem. 67 (2002) 711-719;(l) A. Heqedues, A. Cwik, Z. Hell, et al., Microwave-assisted conversion of oximes into nitriles in the presence of a zeolite, Green Chem. 4 (2002) 618-620;(m) M. Boruah, D. Konwar, AlCl3·6H2O/KI/H2O/CH3CN: a new alternate system for dehydration of oximes and amides in hydrated media, J. Org. Chem. 67 (2002) 7138-7139;(n) M.M. Heravi, S. Sadjadi, R. Hekmatshoar, H.A. Oskooie, F.F. Bamoharram, Dehydration of oximes to nitriles catalyzed by a green heteropolyacid catalyst: Preyssler's anion, [NaP5W30O110]14-, Chin. J. Chem. 27 (2009) 607-609;(o) B. Jose, M.S. Sulatha, P.M. Pillai, S. Prathapan, A new method for the generation of nitriles from aldoximes, Synth. Commun. 30 (2000) 1509-1514;(p) S.S. Chaudhari, K.G. Akananchi, Thionyl chloride-benzotriazole: an efficient system for transformation of aldoximes to nitriles, Synth. Commun. 29 (1999) 1741-1745;(q) N. Iranpoor, H. Firouzabadi, G. Aghapour, A rapid and facile conversion of primary amides and aldoximes to nitriles and ketoximes to amides with triphenylphosphine and N-chlorosuccinimide, Synth. Commun. 32 (2002) 2535-2541;(r) D. Sahs, A. Saha, B.C. Ranu, Ionic liquid-promoted dehydration of aldoximes: a convenient access to aromatic, heteroaromatic and aliphatic nitriles, Tetrahedron Lett. 50 (2009) 6088-6091;(s) H. Veisi, Direct oxidative conversion of alcohols, amines, aldehydes, and benzyl halides into the corresponding nitriles with trichloroisocyanuric acid in aqueous ammonia, Synthesis (2010) 2631-2635;(t) R.G. Kalkhambkar, S.D. Bunge, K.K. Laali, Reaction of triflyl-imidazole with aldoximes: facile synthesis of nitriles and formation of novel aldoxime-bis(Ntriflyl)- imidazole adducts, Tetrahedron Lett. 52 (2011) 5184-5187; (u) R.M. Denton, J. An, P. Lindovska, W. Lewis, Phosphonium salt-catalysed synthesis of nitriles from in situ activated oximes, Tetrahedron Lett. 68 (2012) 2899- 2905; (v) R. Rezaei, M. Karami, Microwave promoted rapid dehydration of aldoximes to nitriles using melamine-formaldehyde resin supported sulphuric acid in dry media, Chin. Chem. Lett. 22 (2011) 815-818. |
| [5] | (a) R. Ghorbani-Vaghei, H. Shahbazi, H. Veisi, Mild bromination of unreactive aromatic compounds, Tetrahedron Lett. 53 (2012) 2325-2327;(b) R. Ghorbani-Vaghei, S. Hajinazari, M. Engashte, Poly(N,N'-dibromo-N-ethylbenzene- 1,3-disulfonamide), N,N,N',N'-tetrabromobenzene-1,3-disulfonamide as new reagents for conjugate addition of indole, pyrrole with α,β-unsaturated ketones, J. Iran. Chem. Soc. 9 (2012) 655-660;(c) R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri, M. Ghavidel, One-pot synthesis of aliphatic and aromatic 2H-indazolo[2,1-b]phthalazine- triones catalyzed by N-halosulfonamides under solvent-free conditions, Tetrahedron 67 (2011) 1930-1937;(d) H. Veisi, R. Ghorbani-Vaghei, J. Mahmoodi, Poly(N,N'-dichloro-N-ethyl-benzene- 1,3-disulfonamide) and N,N,N',N'-tetrachlorobenzene-1,3-disulfonamide as efficient reagents to direct oxidative conversion of thiols and disulfide to sulfonyl chlorides, Bull. Korean Chem. Soc. 32 (2011) 3692-3695;(e) R. Ghorbani-Vaghei, S. Akbari-Dadamahaleh, M. Amiri, Poly(N-bromo-N-ethylbenzene- 1,3-disulfonamide), N,N,N',N'-tetrabromobenzene-1,3-disulfonamide as new efficient reagents for conversion of alcohols to THP ethers and aldehydes to oxazoline compounds, J. Iran. Chem. Soc. 7 (2010) 301-307;(f) R. Ghorbani-Vaghei, H. Veisi, The application of poly(N,N'-dibromo-N-ethylbenzene- 1,3-disulfonamide) and N,N,N',N'-tetrabromobenzene-1,3-disulfonamide as catalysts for one-pot synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles and 1,5-benzodiazepines, and new reagents for synthesis of benzimidazoles, Mol. Divers. 14 (2010) 249-256;(g) R. Ghorbani-Vaghei, M. Amiri, N. Moshfeghifar, H. Veisi, S. Akbari-Dadamahaleh, Poly(N,N'-dibromo-N-ethyl-benzene-1,3-disulfonamide) and N,N,N',N'-tetrabromobenzene- 1,3-disulfonamide as effective catalysts for conversion of aldehydes to 1,1-diacetates and acetals, J. Iran. Chem. Soc. 6 (2009) 754-760;(h) R. Ghorbani-Vaghei, H. Veisi, M. Amiri, Poly(N,N'-dichloro-N-ethyl-benzene- 1,3-disulfonamide), N,N,N',N'-tetrachlorobenzene-1,3-disulfonamide, poly(N,N'- dibromo-N-ethyl-benzene-1,3-disulfonamide), and N,N,N',N'-tetrabromobenzene- 1,3-disulfonamide catalyzed formylation of amines and alcohols using ethyl formate under microwave irradiation, J. Iran. Chem. Soc. 6 (2009) 761-768;(i) R. Ghorbani-Vaghei, M. Chegini, H. Veisi, M. Karimi-Tabar, Poly(N,N'-dibromo-Nethyl- benzene-1,3-disulfonamide), N,N,N',N'-tetrabromobenzene-1,3-disulfonamide and novel poly(N,N,N'-dibromo-N-phenylbenzene-1,3-disulfonamide) as powerful reagents for benzylicbromination, Tetrahedron Lett. 50 (2009) 1861-1865;(j) R. Ghorbani-Vaghei, S. Akbari-Dadamahaleh, Poly(N-bromo-N-ethylbenzene- 1,3-disulfonamide) and N,N,N',N'-tetrabromobenzene-1,3-disulfonamide as efficient reagents for synthesis of quinolines, Tetrahedron Lett. 50 (2009) 1055-1058;(k) R. Ghorbani-Vaghei, H. Veisi, Poly(N,N,N'-dichloro-N-ethylbenzene-1,3-disulfonamide) andN,N,N',N'-tetrachlorobenzene-1,3-disulfonamide as novel reagents for the synthesis of N-chloroamines, nitriles and aldehydes, Synthesis (2009) 945-950;(l) R. Ghorbani-Vaghei, M.A. Zolfigol, M. Amiri, H. Veisi, N,N,N',N'-Tetrabromobenzene- 1,3-disulfonamide and poly(N-bromo-N-ethyl-benzene-1,3-disulfonamide) as efficient catalysts for the methoxymethylation of alcohols under solvent-free conditions, J. Chin. Chem. Soc. 55 (2008) 632-635;(m) R.Ghorbani-Vaghei, H. Veisi,M.Amiri,Microwave-assisted oxidationof alcohols with N,N,N',N'-tetrabromobenzene-1,3-disulfonamide and poly(n-bromobenzene- 1,3-disulfonamide) under solvent-free conditions, J. Chin. Chem. Soc. 54 (2007) 1257-1260;(n) R. Ghorbani-Vaghei, M.A. Zolfigol, M. Chegeny, H. Veisi, Poly(N-bromobenzene- 1,3-disulfonamide) and N,N,N',N'-tetrabromobenzene-1,3-disulfonamide as novel catalytic reagents for silylation of alcohols, phenols, and thiols using hexamethyldisilazane, Tetrahedron Lett. 47 (2006) 4505-4508;(o) R. Ghorbani-Vaghei, H. Jalili, Mild and regioselective bromination of aromatic compounds with N,N,N',N'-tetrabromobenzene-1,3-disulfonamide and poly(N-bromobenzene- 1,3-disulfonylamide), Synthesis (2005) 1099-1102;(p) R. Ghorbani-Vaghei, E. Shahbazee, Facile and mild deprotection of semicarbazones under solvent-free conditions with N,N,N',N'-tetrabromo-benzene-1,3-disulfonylamide, J. Braz. Chem. Soc. 16 (2005) 647-649;(q) R. Ghorbani-Vaghei, E. Shahbazee, H. Veisi, |
| [6] | D.R. Kirklin, E.S. Domalski, Enthalpies of combustion of triphenylphosphine and triphenylphosphine oxide, J. Chem. Thermodyn. (1988) 743-754. |
| [7] | (a) D.S. Bhalerao, U.S. Mahajan, K.H. Chaudhari, K.G. Akamanchi, o-Iodoxybenzoic acid- and tetraethylammonium bromide-mediated oxidative transformation of primary carboxamides to one-carbon dehomologated nitriles, J. Org. Chem. 72 (2007) 662-665;(b) Online Data from Product Catalog, Sigma-Aldrich, 2009 http://www.sigmaaldrich. com/technical-service-home/product-catalog.html;(c) S. Talukdar, J.L. Hsu, T.C. Chou, J.M. Fang, Direct transformation of aldehydes to nitriles using iodine in ammonia water, Tetrahedron Lett. 42 (2001) 1103-1105;(d) D.R. Lide, CRC Handbook of Chemistry and Physics, 87th ed., CRC Press, Boca Raton, FL, 2006;(e) H. Sharghi, M.H. Sarvari, Graphite as an efficient catalyst for one-step conversion of aldehydes into nitriles in dry media, Synthesis (2003) 243-246;(f) S. Ushijima, K. Moriyama, H. Togo, Practical one-pot transformation of electronrich aromatics into aromatic nitriles with molecular iodine and aq NH3 using Vilsmeier-Haack reaction, Tetrahedron 68 (2012) 4588-4595;(g) P. Capdevielle, A. Lavigne, M. Maumy, Improved and extended one-step conversion of primary amines into nitriles by copper-catalyzed oxidation, Synthesis (1989) 453-454;(h) H.M. Sampath Kumar, P.K. Mohanty, M. Suresh Kumar, J.S. Yadav, Microwave promoted rapid dehydration of aldoximes to nitriles on a solid support, Synth. Commun. 27 (1997) 1327-1333;(i) S. Iida, H. Togo, Direct oxidative conversion of alcohols and amines to nitriles with molecular iodine and DIH in aq NH3, Tetrahedron 63 (2007) 8274-8281;(j) B.P. Bandgar, S.S. Makone, Organic reactions in water: transformation of aldehydes to nitriles using NBS under mild conditions, Synth. Commun. 36 (2006) 1347-1352;(k) Z. Rappaport, CRC Handbook of Tables for Organic Compound Identification, 3rd ed., The Chemical Rubber, USA, 1967. |