The extracts of Ginkgo biloba leaves are widely used in traditional Chinese medicines and dietary supplements to improve peripheral blood flow condition and reduce cerebral insufficiency. The flavonoids and terpene lactones are considered to be the main beneficial components. Over 30 flavonoids,including flavonol glycosides,flavonols,flavones,biflavones and catechins,have been isolated from G. biloba leaves [1]. G. biloba flavonoids have been prescribed for sensitivity-related chronic cerebral deterioration and functional disturbances,as well as cerebral vascular accidents and cranial trauma [2]. Recently,increasing attention has been directed to G. biloba flavonoids,due to their various antiinflammatory and antitumor activities [3, 4]. Thus,identification and qualitative analysis of the indigenous flavonoids are crucial to authentication of G. biloba leaves and their products.
Because their surface can be easily modified with functional groups and they can be magnetically separated fromsolution,Fe3O4 magnetic nanoparticles (MNPs) have been widely applied in solid phase extraction of small molecules in mixtures. Human serum albumin (HSA) is the most abundant protein in blood plasma and playsmajor roles in the transportationand deposition of endogenous and exogenous ligands [5]. Therefore,the use of HSA immobilized on the surface ofMNPs can be of help to screen bioactive compounds in herbs [6, 7]. In this novel approach,HSA-MNPs were used to investigate the compounds presented in the leaves of G. biloba,and all the compounds extracted by HSA-MNPs were identified as flavonoids with HPLC-MS. It was demonstrated that HSA-MNPs coupled with HPLC-MS is an effective and convenient way to screen flavonoids from botanical extracts.
Fe3O4 MNPs were synthesized following the reported method with some modifications [8]. FeCl36H2O,FeCl24H2O,sodium oleate and sodium hydroxide were mixed in toluene/ethanol/ water mixture solvent and refluxed at 74 ℃ for 4 h. The SiO2- coated MNPs were first produced in an optimized inverse microemulsion Triton-X100/hexanol/water/cyclohexane system with the MNPs obtained from the above method. Aqueous ammonia (28-30 wt%),tetraethyl orthosilicate (TEOS) and 3- aminopropyltrimethoxysilane (APTMS) were added to the waterin- oil microemulsion to form the amino-functionalized MNPs [9]. The resultant particles were then dispensed in 5% glutaraldehyde solution to provide -CHO to the silica coating. Finally,the aldehyde functionalized MNPs were incubated with HSA to obtain HSAfunctionalized MNPs. The nanoparticles were suspended in NH4OAc solution (10 mmol/L,pH 7.4) and kept at 4 ℃ until use.
Dried G. biloba leaves (2.0 g) were sliced and then sonicated in 50 mL 95% ethanol for 1 h. The decoction was evaporated to give a dry extract,which was re-dissolved in 10 mL water. After being filtered and centrifuged,the supernatant was kept aside for extraction with HSA-MNPs. The 0.5 mL HSA-MNPs suspension was added to 1 mL of the above supernatant in a micro-centrifuge tube. The mixture was shaken at room temperature for 30 min on a vortex oscillator and separated by an out field magnet. The MNPs were washed three times with 2 mL of NH4OAc solution. 1 mL of 50% ACN was added and shaken for 2 min to elute the compounds bound with HSA. Finally,the eluent was stored at 4 ℃ for HPLC-MS analysis.
Chromatographic analyses were performed on an Agilent 1100 Series HPLC system (Agilent,Palo Alto,CA) coupled with photo diode array (PDA) detector and a Diamonsil C18 column (250 mm × 4.6 mm,5 μm). A flow rate of 1.0 mL/min was employed with a solvent gradient of 10%-30% B in 60 min (solvent A: 0.1% acetic acid/water; solvent B: acetonitrile). A high resolution electrospray mass spectrometer (MicrOTOF-Q II,Bruker Daltonics Corporation,USA) was operated in negative ion mode for HPLC- MS. The capillary voltage was set at 4.0 kV with an end plate offset at 500 V. Data was collected from 50 m/z to 3000 m/z,and the dry gas was set to 4.0 L/min at 180 8C with a nebulization gas pressure of 0.3 bar.
ThermoQuest Finnigan LCQDECA system equipped with an electrospray ionization source (ThermoQuest LC/MS Division, San Jose,CA,USA) was used for mass spectrometric analysis. The operating conditions were optimized in negative mode as following: sheath gas flow rate,35 units; auxiliary gas flow rate,0 units; capillary temperature,250 ℃; capillary voltage,4.5 kV. Samples were introduced into the ESI source by continuous infusion at a flow rate of 5 μL/min using a syringe pump
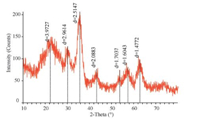
We have made some slight modifications when fabricating the amino-functionalized MNPs. Transmission electron microscopy (TEM) observation indicates that the amino-functionalized MNPs with core-shell structure were spherical and uniform,as shown in Fig. 1. The X-ray diffraction (XRD) pattern of amino-functionalized MNPs is shown in Fig. 2. This spectrum,except for a peak around 2θ = 25° attributed to amorphous phase of silica,was in good agreement with the Fe3O4 phase,indicating that the particles were composed of Fe3O4[10].

|
Download:
|
| Fig.1 TEM image of amino-functionalized MNPs. | |
|
Download:
|
| Fig.2 XRD patterns of amino-functionalized MNPs. | |
Fig. 3 displays the chromatograms of G. biloba leaves extract and the 50% ACN eluent from HSA-MNPs after the extraction process. Although many peaks appeared in the chromatograms of G. biloba leaf extract,9 peaks marked with the numbers 1-9 could be clearly found in the eluent. By analysis of theUVspectra,compounds 1-9 all typically had a maximum absorbance near 260 nm and 350 nm, whichwere the typical spectra of flavonoids in G. biloba [11]. In order to identify these 9 compounds,the HPLC-MS experiment was carried out. With the reference of the known compounds isolated from leaves of G. biloba,their molecular formulas were identified by the high resolution ESI-Q-TOFMS. Furthermore,the structural elucidation was carried out by MS2 (shown in Fig. 4) of each compound.

|
Download:
|
| Fig.3The UV chromatograms at 280 nm of G. biloba leaves extract (A) and the 50% ACN eluent from HSA-MNPs after the extraction process (B). | |

|
Download:
|
| Fig.4ESI-MSn spectra of graphic peaks in the eluent,m/z 609 (1),463 (2),593 (3),623 (4),447 (5),477 (6),755 (8),739 (9),respectively. | |
In the negative mode,all the flavonoids revealed quasimolecular ions [MH] in the MS spectrum. Ginkgo flavonol glycosides are a group of small complex molecules that can be hydrolyzed to give aglycones of kaempferol,quercetin or isorhamnetin [12]. Upon fragmentation,the glycosides lost the bound sugar groups consecutively and produced the corresponding aglycones; and the sugar groups were glucosyl,rhamnosyl and coumaroyl groups. There were two main peaks attributed to compounds 8 and 9 in the HPLC chromatograms of the 50% ACN eluent from HSA-MNPs after the extraction process. The molecular formula of compound 8 was determined to be C36H35O18 by high resolution mass spectrum (m/z 755.1853). The MS2 spectrum of compound 8 gave fragment ions at m/z 609,indicating the loss of a coumaroyl group. The MS3 spectrum of the m/z 609 showed fragment ions at m/z 447 and 300,which indicated a loss of the glucosyl moiety. By comparison of fragmentation pathways with those reported in the literatures,compound 8 was characterized as 3-O-{2-O-[6-O-(p-coumaroyl)-β-D-glucosyl]-α-L-rhamnosyl} quercetin. The molecular formula of compound 9 was determined to be C36H35O17 by high resolution MS (m/z 739.1841). The MS2 spectrum of the compound 9 showed fragment ions atm/z 593,indicating the first group to be lost was a coumaroyl group. The MS3 spectrum of them/z 593 showed fragment ions atm/z 431, which indicated a loss of the glucosyl moiety. Therefore,compound 9 was characterized as 3-O-{2-O-[6-O-(p-coumaroyl)-β-D-glucosyl]- α-L-rhamnosyl} kaempferol [13, 14].
In addition to the two main peaks,the other seven flavonoids were tentatively elucidated with high resolution mass and MS2 experiment. TheMS/MS spectrum of the m/z 609 (peak 1,HR-MS for 609.1435,C27H29O16) showed fragment ions at m/z 447,429,and 301,which are different from rutin,which possesses the same molecular weight [15]. The fragment ion atm/z 447 indicated a loss of the glucosyl moiety. By comparison with the published literature [16, 17],peak 1 was tentatively identified as 3-O-[2-O-(β-Dglucosyl)- α-L-rhamnosyl] quercetin,which is an isomer of rutin. Similarly,the fragment ions of compound 3 (HR-MS for 593.1489, C27H29O15) were 431,413,and 285,so peak 3 was identified as 3-O- [2-O-(β-D-glucosyl)-α-L-rhamnosyl] kaempferol [14]. Fragmentation of compound 4 (HR-MSfor 623.1598,C28H31O16) yielded an ion of m/z 315,corresponding to the loss of 6-O-(α-L-rhamnosyl)-β-Dglucosylmoiety [13].Thus,compound 4 was tentatively identifiedas 3-O-[6-O-(α-L-rhamnosyl)-β-D-glucosyl] isorhamnetin.
The MS/MS spectrum of the m/z 463 (peak 2,HR-MS for 463.0874,C21H19O12) gave a fragment ion at m/z 301,representing a loss of a glucosyl unit. Therefore,peak 2 was tentatively identified as 3-O-(β-D-glucosyl)quercetin [14]. The MS/MS spectrum of the m/z 447 (peak 5,HR-MS for 447.0910,C21H19O11) showed a fragment ion of m/z 285,suggesting it was a kaempferol glycoside like compound 2. Thus,compound 5 was identified as 3-O-(β-Dglucosyl) kaempferol. In the same manner [14],the m/z 477 of compound 6 (HR-MSfor 477.1026,C22H21O12) gave fragment ions at m/z 315,resulting in the identification of 3-O-(β-D-glucosyl) isorhamnetin toward compound 6 [13]. The molecular formula of m/z 287.0551 (peak 7) is C15H11O6,determined by high resolution mass spectrum. Itwas suspected tobe a flavonoid linked tounknown aglycone [18],and its structure needs to be further identified.
Compared with the routine phytochemical procedure,extraction using HSA-MNPs is an exceptionally convenient method to identify small bioactive molecules from botanical extracts [19]. HSA,the most abundant protein in blood plasma,is an important drug carrier protein and has been used as a model protein for diverse biophysical and physiochemical studies [20]. Recently,the molecular property-affinity relationship of flavonoids for HSA in vitro was described. The binding process between flavonoids and HSA was investigated through binding constant,binding affinity, binding distance and energy transfer,which were measured by various spectroscopic methods. The structural differences among flavonoids affect their affinities to HSA significantly. For example, methylation of hydroxyl groups in flavonoids enhanced the binding affinities for HSA,and hydroxylation and glycosylation on rings A,B,and C of flavonoids also affected the affinity for HSA [21]. The nine flavonoids which were selectively extracted from the extract of G. biloba leaves by HSA-MNPs,are the possible ligands of HSA and showed potential bioactivities.
The chemical variety of the constituents in G. biloba leaves is so rich that it is hard to isolate each compound of interest for analysis. Although a lot of compounds have been purified by tedious chromatographic separation process,many of them were useless as far as bioactivity is concerned. In this work,with the selective extraction of HSA-MNPs,flavonoids were enriched while other types of compounds were removed. Here,HSA-MNPs combined with HPLC-MS could be used for qualitative analysis and authentication of G. biloba leaves and their products. This method may be further applied as a guide for separating bioactive flavonoids conveniently and effectively from traditional Chinese
Financial support from National Natural Science Foundation of China (Nos. 21072184 and 81173536) is gratefully acknowledged.
| [1] | L.Z. Lin, P. Chen, M. Ozcan, J.M. Harnly, Chromatographic profiles and identification of new phenolic components of Ginkgo biloba leaves and selected products, J. Agric. Food Chem. 56 (2008) 6671-6679. |
| [2] | K.L. Chiu, Y.C. Cheng, J.H. Chen, C.M.J. Chang, P.W. Yang, Supercritical fluids extraction of Ginkgo ginkgolides and flavonoids, J. Supercrit. Fluids 24 (2002) 77-87. |
| [3] | C. Gaudineau, R. Beckerman, S. Welbourn, K. Auclair, Inhibition of human P450 enzymes by multiple constituents of the Ginkgo biloba extract, Biochem. Biophys. Res. Commun. 318 (2004) 1072-1078. |
| [4] | T.L.Wadsworth, T.L. McDonald, D.R. Koop, Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide induced signaling pathways involved in the release of tumor necrosis factor alpha, Biochem. Pharmacol. 62 (2001) 963-974. |
| [5] | K. Yamasaki, T. Maruyama, U. KraghHansen, M. Otagiri, Characterization of site I on human serum albumin: concept about the structure of a drug binding site, Biophys. Acta 1295 (1996) 147-157. |
| [6] | L.S. Qing, Y. Xue, W.L. Deng, et al., Ligand fishing with functionalized magnetic nanoparticles coupled with mass spectrometry for herbal medicine analysis, Anal. Bioanal. Chem. 399 (2011) 1223-1231. |
| [7] | L.S. Qing, Y. Xue, Y. Zheng, et al., Ligand fishing from Dioscorea nipponica extract using human serum albumin functionalized magnetic nanoparticles, J. Chromatogr. A 1217 (2010) 4663-4668. |
| [8] | X. Wen, J. Yang, B. He, Z. Gu, Preparation of monodisperse magnetite nanoparticles under mild conditions, Curr. Appl. Phys. 8 (2008) 535-541. |
| [9] | C. Vogt, M.S. Toprak, M. Muhammed, et al., High quality and tuneable silica shell magnetic core nanoparticles, J. Nanopart. Res. 12 (2010) 1137-1147. |
| [10] | B. Liu, W.X. Xie, D.P. Wang, et al., Preparation and characterization of magnetic luminescent nanocomposite particles, Mater. Lett. 62 (2008) 3014-3017. |
| [11] | Y.B. Ji, Q.S. Xu, Y.Z. Hu, Y.V. Heyden, Development, optimization and validation of a fingerprint of Ginkgo biloba extracts by high-performance liquid chromatography, J. Chromatogr. A 1066 (2005) 97-104. |
| [12] | L. Zhang, Z.X. Xiang, Determination of the flavonoids from Ginkgo biloba extract by high performance liquid chromatography, Chin. Chem. Lett. (2002) 968-970. |
| [13] | J.J. Song, G.Z. Fang, Y. Zhang, Q.L. Deng, S. Wang, Fingerprint analysis of Ginkgo biloba leaves and related health foods by high performance liquid chromatography/ electrospray ionization mass spectrometry, J. AOAC Int. 93 (2010) 1798-1805. |
| [14] | X. Yao, G. Zhou, Y. Tang, et al., UPLC-PDA-TOF/MS coupled with multivariate statistical analysis to rapidly analyze and evaluate Ginkgo biloba leaves from different origin, Drug Test Analysis (2013). |
| [15] | M. Dubber, V. Sewram, N. Mshicileli, G.S. Shephard, I. Kanfer, The simultaneous determination of selected flavonol glycosides and aglycones in Ginkgo biloba oral dosage forms by high performance liquid chromatography-electrospray ionisation mass spectrometry, J. Pharmaceut. Biomed. 37 (2005) 723-731. |
| [16] | X.P. Ding, J. Qi, Y.X. Chang, et al., Quality control of flavonoids in Ginkgo biloba leaves by high-performance liquid chromatography with diode array detection and on line radical scavenging activity detection, J. Chromatogr. A 1216 (2009) 2204-2210. |
| [17] | A. Hasler, O. Sticher, B. Meier, Identification and determination of the flavonoids from Ginkgo biloba by high performance liquid chromatography, J. Chromatogr. 605 (1992) 41-48. |
| [18] | S. Ding, E. Dudley, S. Plummer, et al., Fingerprint profile of Ginkgo biloba nutritional supplements by LC/ESI-MS/MS, Phytochemistry 69 (2008) 1555-1564. |
| [19] | L.S. Qing, X.Q. Shan, X.M. Xu, et al., Rapid probe and isolation of bioactive compounds from Dioscorea panthaica using human serum albumin functionalized magnetic nano-particles (HSA-MNPs) based ligand fishing coupled with electrospray ionization mass spectrometry, Rapid Commun. Mass Spectrom. 24 (2010) 3335-3339. |
| [20] | Z. Jurasekova, G. Marconi, S. Sanchez-Cortes, A. Torreggiani, Spectroscopic and molecular modeling studies on the binding of the flavonoid luteolin and human serum albumin, Biopolymers 91 (2009) 917-927. |
| [21] | J.B. Xiao, T.T. Chen, H. Cao, L.S. Chen, F. Yang, Molecular property affinity relationship of flavanoids and flavonoids for HSA in vitro, Mol. Nutr. Food Res. 55 (2011) 310-317. |




