Lignin is one of the most abundant renewable resources in nature,and the efficient utilization of the residual lignin from hydrolyzed biomass,pulp,and paper industry is of great significance to solve the problems of environment and resources. However,compared to other biomasses,the utilization of lignin is limited due to its complicated chemical structure and the corresponding chemical reaction mechanism. Currently,lignin is generally assumed to be an amorphous three-dimensional net polymer composed of three phenylpropane units that are linked by β-O-4,α-O-4,β-β,β-5,β-1,5-5 or 4-O-5 chemical bonds. In order to obtain the principle of bond cleavage and combination in the chemical reactions of lignin,lignin model compounds are usually used to provide a theoretical foundation for the more efficient utilization of lignin.
Creosol was used as monomeric lignin model compound to study its chemical reactions [1]. Meanwhile,a wide variety of lignin model compounds were synthesized,including dimer [2, 3], trimer [4, 5, 6, 7],tetramer [8],hexamer [9] and polymers [10, 11],most of which contained only β-O-4 linkage. Although the β-O-4 linkage is themost abundant and probably themostimportant substructure in lignin,the lignin model compounds containing only β-O-4 linkage cannot reflect the entire chemical reaction characteristics of lignin. In addition,the yields of lignin model compounds preparation are very low. Landucci et al. [12] prepared a dimeric lignin model compound containing a β-aryl ether bond with a total yield of 9% using a ten-step sequence. Therefore,there is an increasing interest in obtaining an oligomeric lignin model compound containing several interunit linkages. The α-O-4 linkage is also an important chemical bond linkage in the lignin structure. In the presentwork,3- methoxy-4-benzyloxy-α-(3-methoxy-4-(1-propenyl)phenol)-acetophenonewas synthesized under microwave irradiation,and it may be used as a trimeric lignin model compound containing α-O-4 and β-O-4 chemical bonds linkage.
All reagents in this work were of analytical grade. Benzyl bromide,acetovanillone and isoeugenol were provided by Aladdin Co.,China. MS measurements were carried out by an MS Trace DSQ (Finnigan Inc.,American) equipped with a quadrupole MS detector,The MS detector was kept at 473 K. 1H NMR and 13C NMR spectra were recorded with a Bruker DRX-400 spectrometer (Bruker,Germany). Deuterated chloroform (CDCl3) was used as solvent.
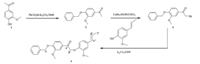
The synthetic route was demonstrated in Scheme 1
|
Download:
|
| Scheme 1 Synthesis of lignin model compound. | |
0.830 g (5 mmol) of acetovanillone (1),1.026 g (6 mmol) of benzyl bromide,0.828 g (6 mmol) of powdered potassium carbonate,and 10 mL of dimethyl formamide were added to a microwave accelerated reaction system (Milstone Ethos A,Italy). After reacting for 15 min at 80 ℃,the reaction mixture was poured into 30 mL of water and white precipitates appeared. The precipitate was collected and washed with distilled water several times and then dried at room temperature under vacuum to give compound 2 in 95.31% yield. 1H NMR (400 MHz,CDCl3): δ 2.57 (s, 3H,CH3),3.95 (s,3H,OCH3),5.25 (s,2H,CH2),6.90-7.57 (m,8H, aromatics); 13C NMR (100 MHz,CDCl3):δ 26.3 (CH3),56.1 (OCH3), 70.8 (CH2),112.2-152.3 (C,aromatics),197.0 (C=O).
1.565 g (7 mmol) of cooper bromide in 12 mL ethanol was added dropwise to a reactor containing 0.768 g (3 mmol) of compound 2 in chloroform (3 mL). The reaction was carried out at 70℃ for 15 min under themicrowave irradiation. The reaction mixture was poured into a sodium chloride aqueous solution after solvent was removed by vacuum distillation,and then the crude product was extracted with ethyl acetate twice and dried over MgSO4 and evaporated under vacuum. The resulting product was further purified by column chromatography with silica gel using dichlomethane/n-hexane (10/3,v/v) as the mobile phase. Thin layer chromatography was used to identify the product,and the purified compound 3 was obtained after evaporation under vacuum in 87.3% yield. 1H NMR (400 MHz, CDCl3): δ 3.95 (s,3H,OCH3),4.47 (s,2H,CH2Br),5.24 (s,2H,CH2), 6.50-7.80 (m,8H,aromatics); 13C NMR (100 MHz,CDCl3): δ 30.6 (CH2Br),56.6 (OCH3),71.1 (CH2),112.2-153.5 (C,aromatics), 190.3 (C=O).
0.720 g (2.15 mmol) compound 3,0.492 g (3 mmol) isoeugenol, and 0.414 g (3 mmol) powdered potassium carbonate in 10 mL dimethyl formamide were added to a microwave accelerated reaction system. After reacting at 80 ℃ for 30min, the reaction mixture was purified using the same processes of compound 3. The resulting yellow crystals were washed with a small amount of petroleum ether and dried at 25 ℃ under vacuum to give compound 4 in 90.6% yield. 1H NMR (400 MHz, CDCl3): δ 1.83 (s,3H,CH3),3.86-3.92 (2 s,6H,OCH3),5.21-5.24 (2 s,4H,CH2),6.06-6.13 (m,1H,Hβ"),6.28-6.33 (m,1 H,Hα"), 6.72-6.89 (m,4 H,aromatics),7.29-7.60 (m,7 H,aromatics); 13C NMR (100 MHz,CDCl3): δ 18.5(Cg),56.1 (2 OCH3),70.8 (Cα), 72.2 (Cβ'),122.9 (Cβ"),128.5 (Cα"),109.4.7-152.9 (C,aromatics), 193.3 (Cα').
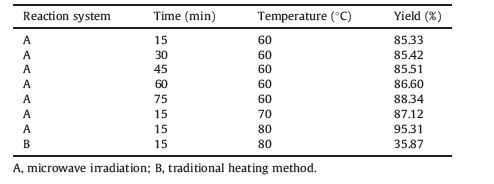
The effects of microwave irradiation on the reaction time and temperature for the synthesis of 3-methoxy-4-benzyloxyacetophenone (2) are shown in Table 1. The results indicate that when the reaction time is only 15 min,the yield reaches more than 85%. Conversely,the similar reaction heated with a conventional water bath requires 3-4 h to give only a yield of 76% [11, 12]. Table 1 also shows that with longer reaction time,the yield is not noticeably improved. With five times longer reaction time, the yield only increased by 3%. Compared to the conventional heating method,microwave irradiation results in an almost 60% increase in the yield under the same condition.
| Table 1 The influence of time and temperature on the yield of 3-methoxy-4-benzyloxyacetophenone |
Microwave irradiation not only shortens the reaction time,but also significantly improves the yield of the desired products. This may be due to both thermal and specific non-thermal effects of microwave irradiation in chemical reactions. Basically,the rate acceleration is ascribed to the increase of the pre-exponential factor in the Arrhenius equation [13] and the decrease of the activation energy due to polarization of the solvent molecule, resulting in an energy transfer between microwave energy and molecular energy [14].
MS spectrum of compound 4 is shown in Fig. 1. The retention time of the target product is 16.48 min and the m/z is 418.04,which is consistent with the molecule weight of compound 4.

|
Download:
|
| Fig 1 MS spectrum of compound (4). | |
The assignment of signals in the 1H NMR spectrum of compound 4 is shown in Fig. 2. Compared to the NMR spectrum of acetovanillone (1),a CH2 in α-O-4 signal at 5.25-5.29 ppm appears in compound 2,which means that benzyl is induced in compound 2 to form an α-O-4 linkage. It can be found that a CH2Br signal at 4.47 ppm appeared and the CH3 signal at 2.57 ppm disappeared in the compound 3,indicating that the bromination of compound 2 occurs at the methyl group connected to the carbonyl group. After the nucleophilic substitution reaction,the signal of CH2Br at 4.47 ppm in compound 3 disappears because bromine is substituted by Guaiacyl to form a β-O-4 linkage. However,it cannot discriminate between α-O-4 and β-O-4 in the 1H NMR spectrum. And the assignment of signals in the 13C NMR spectrum of compound 4 (Fig. 2) shows that signals at 70.8,72.1,56.1 and 193.3 ppm correspond to Cα and Cβ" in α-O-4 and β-O-4 linkage,methoxyl group and carbonyl group of compound 4,respectively. These results reflect that compound 4 contains some typical structural characteristics,including α-O-4,β-O-4 linkage and guaiacyl structure (3-methoxy-4-hydroxy phenyl propane); therefore,it can be used as a trimeric lignin model compound.

|
Download:
|
| Fig 2 1H NMR spectrum and 13C NMR spectrum of compound 4. | |
A lignin model compound composed of 3 guaiacyl structural units linked by α-O-4 and β-O-4 ether bonds was synthesized via a three-step sequence assisted by microwave irradiation. Under the condition of microwave irradiation,the reaction time of each step is significantly reduced,and the selectivity for the targeted products is greatly improved. The yields of each step and the overall reaction are 95.31%,87.3%,90.6% and 75.4% (95.31% 87.3% 90.6%),respectively. 3-methoxy-4-benzyloxya-( 3-methoxy-4-(1-propenyl)phenol)-acetophenone (4) represents the predominant structure in lignin,therefore it is more accurate in mimicking the chemical reaction characteristics of lignin than other dimeric lignin model compounds and polymeric model compounds that contain only β-O-4 linkages.
This work is financially supported by the National Basic Research Program of China (No. 2012CB215302),National Natural Science Fundation of China (No. 20876064),and the Guangdong Provincial Natural Science Fund (No. 9151064101000082).
| [1] | S. Yasuda, E. Hamaguchi, Y. Matsushita, H. Goto, T. Imai, Ready chemical conversion of acid hydrolysis lignin into water-soluble lignosulfonate II hydroxymethylation and subsequent sulfonation of phenolized lignin model compounds, J. Wood Sci. 44 (1998) 116-124. |
| [2] | M. Lahtinen, K. Kruus, P. Heinonen, J. Sipila, On the reactions of two fungal laccases differing in their redox potential with lignin model compounds: products and their rate of formation, J. Agric. Food Chem. 57 (2009) 8357-8365. |
| [3] | D.R. Dlmmel, D. Shepard, Synthesis of lignin model dimmers by novel techniques, J. Wood Chem. Technol. 2 (1982) 297-315. |
| [4] | V.L. Alves, M.G. Drumond, G.M. Stefani, Synthesis of new trimeric lignin model compounds containing 5-5' and β-O-4' substructures, and their characterization by 1D and 2D NMR techniques, J. Braz. Chem. Soc. 11 (2000) 467-473. |
| [5] | S. Reale, F. Attanasio, N. Spreti, F. De Angelis, Lignin chemistry: biosynthetic study and structural characterisation of coniferyl alcohol oligomers formed in vitro in a micellar environment, Chem. Eur. J. 16 (2010) 6077-6087. |
| [6] | Y. Kamaya, F. Nakatsubo, T. Higuchi, Synthesis of a trimeric lignin model compound composed of b-O-4 and syringaresinol substructures, J. Jpn. Wood Res. Soc. 26 (1980) 471-475. |
| [7] | J.Ralph,R.M.Ede,A.Wilkins, Synthesisof trimeric ligninmodel compounds composed of β-aryl ether and phenylcoumaran structures, Holzforschung 40 (1986) 23-30. |
| [8] | F. Xi, D.W. Reeve, A.B. Mckaque, Synthesis and chemical structures of tetrameric lignin model compounds, J. Wood Chem. Technol. 16 (1996) 35-46. |
| [9] | I. Kilpelainen, A. Tervila-Wilo, H. Perakyla, J. Matikainen, G. Brunow, Synthesis of hexameric lignin model compounds, Holzforschung 48 (1994) 381-386. |
| [10] | R. Katahira, H. Kamttakahara, T. Takano, F. Nakatsubo, Synthesis of β-O-4 type oligomeric lignin model compound by the nucleophilc addition of carbonion to aldehyde group, J. Wood Sci. 52 (2006) 255-260. |
| [11] | T. Kishimoto, Y. Uraki, M. Ubukata, Easy synthesis of β-O-4 type lignin related polymers, Org. Biomol. Chem. 3 (2005) 1067-1073. |
| [12] | L.L. Landucci, S.A. Geddes, T.K. Kirk, Synthesis of 14C labeled 3-methoxy-4- hydrox-y-α-(2-methoxyphenoxy)-β-hydroxyl-propiophenone, a lignin model compound, Holzforschung 35 (1981) 66-69. |
| [13] | P. Lidström, J. Tierney, B. Wathey, J. Westman, Microwave assisted organic synthesis - a review, Tetrahedron 57 (2001) 9225-9283. |
| [14] | S.V. Rajender, Solvent-free accelerated organic syntheses using microwaves, Pure Appl. Chem. 73 (2001) 193-198. |