Among the multiple heterocyclic moieties of biological and pharmacological interest,the oxazole ring has gained considerable attention in past decades [1]. In particular,2,5-disubstituted oxazoles are important substructures in various natural products [2] and show activity against inflammation [3],diabetes [4], bacterial infection [5],cardiovascular disease [6],and cancer [7]. Accordingly,many strategies have been developed for their synthesis [8]. Robinson-Gabriel oxazole synthesis is a widely used method,but the reaction needs hazardous reagents such as H2SO4,PCl5 or POCl3 [9]. In addition,α-diazoketones,the starting materials of the reaction,are dangerous and not readily commercially available. Gold-mediated homogeneous catalysts,because of their unique properties,have now become one of the hot topics in the organic chemistry [10]. Among various types of gold catalysis, the research of α-oxo gold carbene intermediates has contributed significantly to the diversity and versatility of gold chemistry. Gold carbene intermediates generated via gold-catalyzed activation of C-C multiple bonds and subsequently attacked by a nucleophilic reagent such as imine and alkoxy,has proven to be an exceptionally powerful method to construct new C-C and C-N bonds [11]. Recently,the first efficient intermolecular reaction of gold carbene intermediates,using nitriles as both the reacting partner and the reaction solvent,was reported,offering a generally efficient synthesis of 2,5-disubstituted oxazoles [12]. To the best of our knowledge,research on the synthesis and applications of gold- catalyzed oxazoles has beenvirtually nonexistent inpharmaceutical literature. Herein,we report the gold-catalyzed synthesis of a number of 5-aryl-2-methyloxazoles and their cytotoxic effects against MCF-7,A549 and Hela cancer cell lines in vitro.
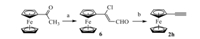
2. ExperimentalThe alkynes 2b-2g,2i were prepared according to the strategy described in Scheme 1 by a modified literature method [13]. In general,reactions of halides 5,the starting materials,with 1.3 equiv. of trimethylsilylacetylene using catalytic quantities of bis(triphenylphosphine) palladium(II) chloride and copper iodide in diisopropyl-amine afforded protected alkynes 3. Hydrolysis of 3 in methanol using anhydrous potassium carbonate led to the formation of the products alkynes 2b-2g,2i,yield 78%-91% after two steps. Ferrocenylacetylene 2h was prepared as shown in Scheme 2 [14]. In the initial step,(2-formy-1-chlorovinyl) ferrocene 6 was synthesized via the reaction of acetylferrocene with Vielsmeier complex prepared from phosphorus oxychloride and DMF in an ice bath. Then the treatment of the alkene 6 with sodium hydroxide solution afforded the corresponding ferroceny- lacetylene 2h.

|
Download:
|
| Scheme 1. Reagents and condition: (a) Pd(PPh3)2Cl2,CuI,(i-Pr)2NH,TMSacetylene; (b) K2CO3,MeOH. | |
|
Download:
|
| Scheme 2. Reagents and condition: (a) POCl3,DMF; (b) NaOH,dioxane,reflux. | |
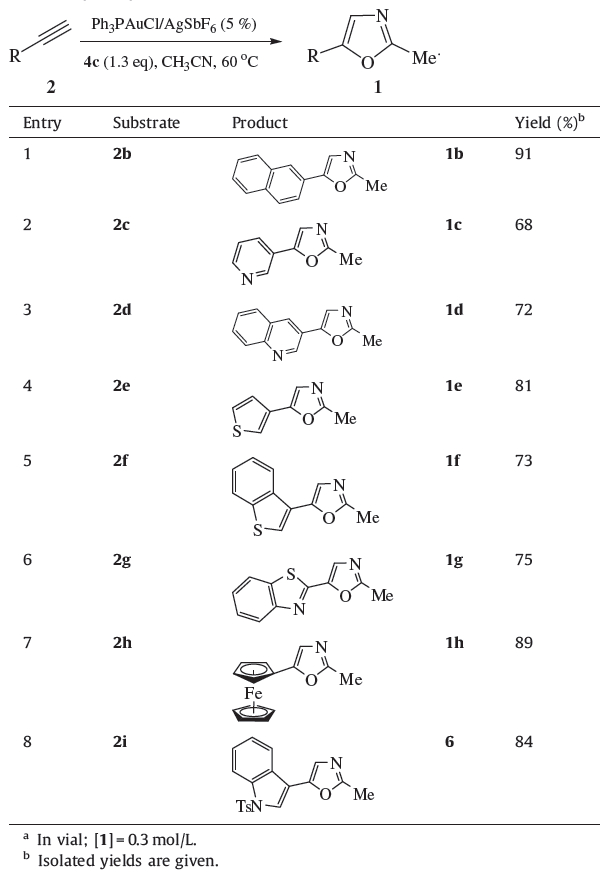
Oxazoles 1 were prepared from alkynes under the reaction conditions of Table 1 and the progress of the reaction was monitored by TLC. Compounds 1 were purified by column chromatography on silica gel in 68%-94% yield. All of them were confirmed by 1H NMR and HRMS spectra [15]. The presence of nitrogen or sulfur in heteroaryl substituents would reduce the activity of gold catalyst.
| Table 1 Gold-catalyzed synthesis of oxazoles 1<.b>.a |
Based on Zhang’s work [12],we further screened the reaction conditions,using phenylacetylene as the substrate. Compared to ambient temperature,the reaction proceeded faster at 60 ℃ (Table 2,entries 1 and 2).
| Table 2 Screening of reaction conditions.a |
Different quinoline N-oxides were tested (Table 2,entries 2-5), 8-ethylquinoline N-oxide (4c) was the best among the oxidants examined. As the use of LAuCl/AgX is becoming more widespread in gold chemistry [16],we envisioned the possibility of increasing the chemical yield of the reaction sequence by examining the roles of the ligand and counterion (Table 2,entries 6-14). Though other gold catalysts (e.g.,Ph3PAuCl/AgNTf2,BrettPhosAuCl/AgNTf2) worked equally well,we chose Ph3PAuCl/AgSbF6 as the catalyst for economic reasons.
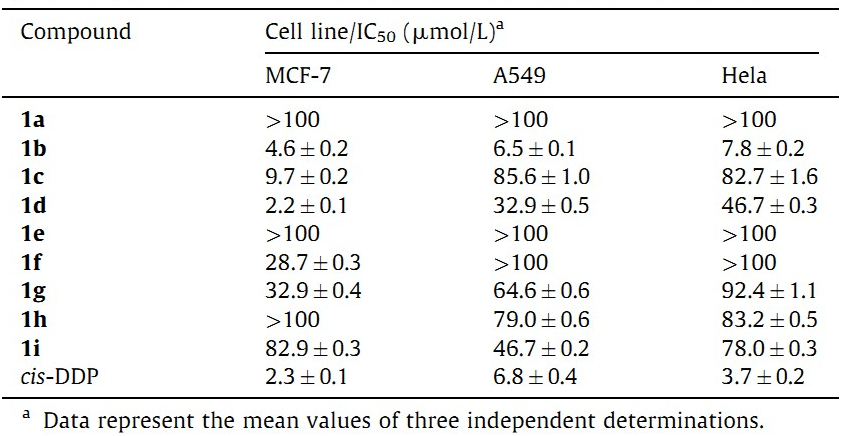
In the screening assay studies,a series of compounds 1a-1i were evaluated against three cell lines: MCF-7 (Human breast carcinoma),A-549 (Human lung carcinoma) and Hela (Human cervical carcinoma). Cis-dichlorodiamineplatinum(II) (cis-DDP) was used as the reference substance. As shown in Table 3, compounds 1b,1c,1d,1f and 1g are the active molecules against various cell lines. The compound 1b exhibited excellent inhibitory activity against all the cancer cell lines and the IC50 values even compared well with cis-DDP.
| Table 3 Cytotoxicity of oxazoles 1a-1i against MCF-7,A549 and Hela cancer cell. |
Introduction of a benzene group increased biological activity,as demonstrated by the IC50 value difference between the pairs 1c and 1d and 1e-1f. It is interesting to note that the compounds with nitrogen heterocyclic groups as the substituents exhibited higher activities than thosewith sulfur heterocyclic groups. The compound 1d with quinoline showed the best selective cytotoxicity against MCF-7cell lineswiththe IC50 values of 2.2 μmol/L,andthe valuewas slightly better than cis-DDP serving as a positive control. Except for 1b,the products exhibited poor activity against Hela and A549 cell lines (more than 30 μmol/L).Usually,ferrocene groups elicit a broad range of biological activities,such as anti-inflammatory,anti- malaria,antimicrobial,anticancer properties [17]. Unfortunately, the compound 1h with a ferrocene group did not exhibit good activity as expected.
4. ConclusionA series of 5-aryl-2-methyloxazoles were prepared and studied for their cytotoxicity against various cancer cell lines. Of the synthesized oxazoles,compound 1b was found to be most cytotoxic against cancer cell lines. In addition,compound 1c and 1d were highly selective against MCF7 cancer cell lines. The structure-activity relationship study showed that naphthalen-2-yl and quinolin-3-yl at the C-5 position of the oxazole ring are important for the activity and selectivity of 5-aryl-2-methylox- azoles.
AcknowledgmentsThis work was supported by NSFC (No. 21276068),Department of Science and Technology Foundation of Hunan Province (No. 2010SK2001) and Hunan Natural Science Foundation (No. 11JJ5008).
| [1] | (a) D. Kumar, M. Kumar, K.H. Chang, et al., An expeditious synthesis and anticancer activity of novel 4-(3'-indolyl)oxazoles, Eur. J. Med. Chem. 45 (2010) 1244- 1249;(b) Z. Jin, Muscarine, imidazole, oxazole, and thiazole alkaloids, Nat. Prod. Rep. 28 (2011) 1143-1191;(c) V.S.C. Yeh, Recent advances in the total syntheses of oxazole-containing natural products, Tetrahedron 60 (2004) 11995-12042;(d) B. Wang, T. Hansen, T. Wang, et al., Total synthesis of phorboxazole A via de novo oxazole formation: strategy and component assembly, J. Am. Chem. Soc. 133 (2011) 1484-1505. |
| [2] | (a) R. Misra, H.Y. Xiao, K. Kim, et al., N-(cycloalkylamino)acyl-2-aminothiazole inhibitors of cyclin-dependent kinase 2. N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl] methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide (BMS-387032), a highly efficacious and selective antitumor agent, J. Med. Chem. 47 (2004) 1719-1728;(b) Y. Koyama, K. Yokose, L. Dolby, et al., Isolation, characterization and synthesis of pimprinine, pimprinethine and pimprinaphine, metabolites of Streptoverticillium olivoreticuli, Agric. Biol. Chem. 45 (1981) 1285-1287. |
| [3] | A.O.D. Souza, M.T.C. Pedrosa, J.B. Alderete, et al., Cytotoxicity, antitumoral and antimycobacterial activity of tetrazole and oxadiazole derivatives, Pharmazie 60 (2005) 396-397. |
| [4] | K. Atul, A. Pervez, M.A. Ram, et al., Novel 2-aryl-naphtho[1,2-d]oxazole derivatives as potential PTP-1B inhibitors showing antihyperglycemic activities, Eur. J. Med. Chem. 44 (2009) 109-116. |
| [5] | L.S. Boulos, M.H.N. Arsanious, E.F. Ewies, Studies on phosphonium Ylides XXV: the behavior of active phosphacumulene and stabilized alkylidenephosphoranes towards 5-(4H)-oxazolones, Phosphorus Sulfur Silicon Relat. Elem. 184 (2009) 275-290. |
| [6] | M. Natesan, Z. Gu, P. Stein, Biphenylsulfonamide endothelin receptor antagonists. 2. Discovery of 4'-oxazolyl biphenylsulfonamides as a new class of potent, highly selective ETA antagonists, J. Med. Chem. 43 (2000) 3111-3117. |
| [7] | W.S. Yang, K. Shimada, D. Delva, et al., Identification of simple compounds with microtubule-binding activity that inhibit cancer cell growth with high potency, ACS Med. Chem. Lett. 3 (2012) 35-38. |
| [8] | I. Cano, E. Álvarez, M.C. Nicasio, et al., Regioselective formation of 2,5-disubstituted oxazoles via copper(i)-catalyzed cycloaddition of acyl azides and 1-alkynes, J. Am. Chem. Soc. 133 (2011) 191-193. |
| [9] | E.E. Wiegand, D.W. Rathburn, Polyphosphoric acid cyclization of acetamidoketones to 2,5-dimethyl-1,3-oxazoles, Synthesis 2 (1970) 648-649. |
| [10] | (a) A.S.K. Hashmi, Gold-catalyzed organic reactions, Chem. Rev. 107 (2007) 3180-3211;(b) A. Corma, A. Leyva-Perez, M. Sabater, et al., Gold-catalyzed carbon-heteroatom bond-forming reactions, Chem. Rev. 111 (2011) 1657-1712;(c) A.S.K. Hashmi, C. Hubbert, Gold and organocatalysis combined, Angew. Chem. Int. Ed. 49 (2010) 1010-1012;(d) B. Alcaide, P. Almendros, J. Alonso, Gold catalyzed oxycyclizations of alkynols and alkyndiols, Org. Biomol. Chem. 9 (2011) 4405-4416. |
| [11] | (a) J. Xiao, X.W. Li, Gold a-oxo carbenoids in catalysis: catalytic oxygen-atom transfer to alkynes, Angew. Chem. Int. Ed. 50 (2011) 7226-7236;(b) B. Lu, C.Q. Li, L.M. Zhang, Gold-catalyzed highly regioselective oxidation of C- C triple bonds without acid additives: propargyl moieties as masked a,b-unsaturated carbonyls, J. Am. Chem. Soc. 132 (2010) 14070-14072;(c) L.W. Ye, W.M. He, L.M. Zhang, A flexible and stereoselective synthesis of azetidin-3-ones through gold-catalyzed intermolecular oxidation of alkynes, Angew. Chem. Int. Ed. 50 (2011) 3236-3239. |
| [12] | W.M. He, C.Q. Li, L.M. Zhang, An efficient [2 + 2 + 1] synthesis of 2,5-disubstituted oxazoles via gold-catalyzed intermolecular alkyne oxidation, J. Am. Chem. Soc. 133 (2011) 8482-8485. |
| [13] | (a) T. Sakamoto, M. Shiraiwa, Y. Konodo, et al., A facile synthesis of ethynylsubstituted six-membered n-heteroaromatic compounds, Synthesis (1983) 312- 314;(b) A. Gangjee, J. Yu, R. Kisliuk, 2-Amino-4-oxo-6-substituted-pyrrolo[2,3-d]pynmidines as potential inhibitors of thymidylate synthase, J. Heterocyclic Chem. 39 (2002) 833-840;(c) J.J. Qi, C.H. Tung, Development of benzothiazole ‘click-on' fluorogenic dyes, Bioorg. Med. Chem. Lett. 21 (2011) 320-323. |
| [14] | (a) M. Rosenblum, N. Brawn, J. Papenmeier, et al., Synthesis of ferrocenylacetylenes, J. Organometal. Chem. 6 (1966) 173-180;(b) A. Aguilar, A. Allen, E. Cabrera, et al., Ferrocenylketene and ferrocenyl-1,2- bisketenes: δirect observation and reactivity measurements, J. Org. Chem. 70 (2005) 9556-9561. |
| [15] | Spectroscopic data: 1a: 1H NMR (400 MHz, CDCl3): δ 2.52 (s, 3H), 7.20 (s, 1H), 7.30 (t, 1H, J = 7.6 Hz), 7.40 (t, 2H, J = 7.6 Hz), 7.60 (d, 2H, J = 7.2 Hz). HRMS-EI: [M+] Calcd. for C10H9NO 159.0679, Found 159.0680. 1b: 1H NMR (400 MHz, CDCl3): δ 2.58 (s, 3H), 7.33 (s, 1H), 7.49 (m, 2H), 7.66 (d, 1H, J = 7.2 Hz), 7.72 (d, 2H, J = 7.2 Hz), 7.87 (d, 1H, J = 7.6 Hz), 8.09 (s, 1H). HRMS-EI: [M+] Calcd. for C14H11NO 209.0836, Found 209.0838. 1c: 1H NMR (400 MHz, CDCl3): δ 2.55 (s, 3H), 7.30 (s, 1H), 7.32-7.36 (m, 1H), 7.87 (d, 1H, J = 8.0 Hz), 8.53 (d, 1H, J = 6.8 Hz), 8.87 (s, 1H). HRMS-EI: [M+] Calcd. for C9H8N2O 160.0632, Found 160.0633. 1d: 1H NMR (400 MHz, CDCl3): δ 2.59 (s, 3H), 7.42 (s, 1H), 7.57 (t, 1H, J = 8.8 Hz), 7.671 (t, 1H, J = 8.2 Hz), 7.85 (d, 1H, J = 8.4 Hz), 8.10 (d, 1H, J = 8.4 Hz), 8.31 (s, 1H), 9.14 (s, 1H). HRMS-EI: [M+] Calcd. for C13H10N2O 210.0788, Found 210.0789. 1e: 1H NMR (400 MHz, CDCl3): δ 2.50 (s, 3H), 7.05 (s, 1H), 7.25-7.27 (m, 1H), 7.35-7.37 (m, 1H), 7.46-7.47 (m, 1H). HRMS-EI: [M+] Calcd. for C8H7NOS 165.0243, Found 165.0242. 1f: 1H NMR (400 MHz, CDCl3): δ 2.51 (s, 3H), 7.32 (s, 1H), 7.40-7.44 (m, 1H), 7.46-7.50 (m, 1H), 7.69 (s, 1H), 7.90 (d, 1H, J = 9.6 Hz), 8.05 (d, 1H, J = 8.0 Hz). HRMS-EI: [M+] Calcd. for C12H9NOS 215.0400, Found 215.0401. 1g: 1H NMR (400 MHz, CDCl3): δ 2.50 (s, 3H), 7.29 (s, 1H), 7.43-7.61 (m, 2H), 7.86 (d, 1H, J = 7.8 Hz), 8.03 (d, 1H, J = 7.4 Hz). HRMS-EI: [M+] Calcd. for C11H8N2OS 216.0352, Found 216.0351. 1h: 1HNMR(400 MHz, CDCl3): δ 2.48 (s, 3H), 4.12 (s, 5H), 4.30 (s, 2H), 4.56 (s, 2H), 6.82 (s, 1H). HRMS-EI: [M+] Calcd. for C14H13FeNO 267.0342, Found 267.0344. Procedure to synthesis of 1i: A solution of NaOH (1.0 mol/L, 3 mL) was added to a stirring solution of 2-methyl-5-(1-tosyl-1H-indol-3-yl)oxazole 6 (106 mg, 0.3 mmol) in 5 mL of methanol and heated to reflux at 80℃ under nitrogen overnight until starting material was consumed as monitored by TLC. Methanol was removed in vacuo and product was extracted with ether (3×20 mL), washed with brine, dried over magnesium sulfate, and concentrated in vacuo to give 56 mg (95% yield) of a yellow solid. 1H NMR (400 MHz, CDCl3): δ 2.58 (s, 3H), 7.16 (s, 1H), 7.22-7.32 (m, 2H), 7.46 (d, 1H, J = 7.6 Hz), 7.52 (d, 1H, J = 7.6 Hz), 7.78 (d, 1H, J = 8.0 Hz), 8.38 (s, 1H). HRMS-EI: [M+] Calcd. for C12H10N2O 198.0788, Found 198.0790. |
| [16] | D.J. Gorin, B.D. Sherry, F.D. Toste, Ligand effects in homogeneous Au catalysis, Chem. Rev. 108 (2008) 3351-3378. |
| [17] | (a) M. Patra, G. Gasser, N. Metzler-Nolte, Small organometallic compounds as antibacterial agents, Dalton Trans. 41 (2012) 6350-6358;(b) R.D. Miao, J. Wei, M.H. Lv, et al., Conjugation of substituted ferrocenyl to thiadiazine as apoptosis-inducing agents targeting the Bax/Bcl-2 pathway, Eur. J. Med. Chem. 46 (2011) 5000-5009;(c) B.H. Long, C.L. He, Y.B. Yang, et al., Synthesis, characterization and antibacterial activities of some new ferrocene-containing penems, Eur. J. Med. Chem. 45 (2010) 1181-1188;(d) B.H. Long, S.Z. Liang, D.C. Xin, et al., Synthesis, characterization and in vitro antiproliferative activities of new 13-cis-retinoyl ferrocene derivatives, Eur. J. Med. Chem. 44 (2009) 2572-2576;(e) S.L. Shen, J. Zhu, M. Li, et al., Synthesis of ferrocenyl pyrazole-containing chiral aminoethanol derivatives and their inhibition against A549 and H322 lung cancer cells, Eur. J. Med. Chem. 54 (2012) 287-294. |