Though no drugs sourced from marine fungi have been approved yet [1],several marine fungal secondary metabolites were found to show good bioactivities [1, 2, 3, 4]. Phenylahistin,a diketopiperazine (DKP) isolated from the cultures of the marine- derived fungus Aspergillus ustus [4],showed cytotoxicity against human cancer cells and inhibition of tubulin polymerization [4]. One of the derivatives of phenylahistin,plinabulin,is now in phase II clinical trials for the treatment of human advanced non-small cell lung cancer as a tumor vascular disrupting agent [3].
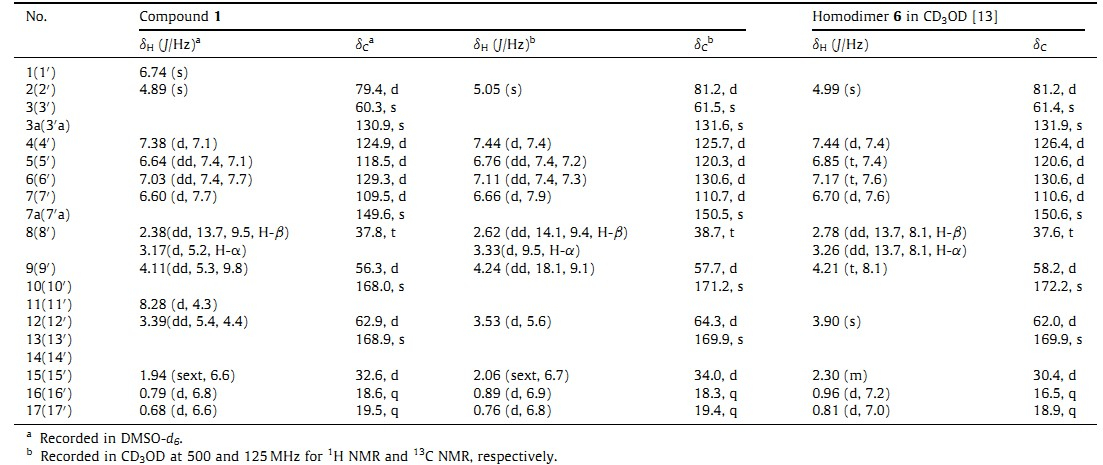
As a part of our ongoing research on bioactive compounds of marine fungal origin with new structures [5],a marine fungal strain identified as Eurotium herbariorum HT-2 was isolated from the marine green alga Enteromorpha prolifera [6]. The ethyl acetate (EtOAc) extract of the fermentation broth of E. herbariorum HT-2 showed antibacterial activity against Enterobacter aerogenes. E. prolifera has occurred in the widespread green tides every summer in Qingdao,China since 2007. Although E. prolifera had been reported to produce rich amino acids,polysaccharides and other nutrients,only a few secondary metabolites,such as cholesterol and chlorophyll-related compounds [7],and pheophytin with potent anti-inflammatory effects [8] were identified from E. prolifera. Except for our previous report on seven DKPs and three benzaldehyde derivatives [6],only one paper reported the secondary metabolites of E. herbariorum: neoechinulins A and B, epiheveadride,isotetrahydroauroglaucin questin,flavoglaucin, cladosporin,and auroglaucin [9]. Further chemical investigation led to identification of a new pyrrolidinoindoline DKP dimmer that we named cristatumin E (1) (Fig. 1). Cristatumin E is an analog of chaetocin,having a bridged disulphide DKP core,with antibacterial and cytostatic activities [10, 11]. Here,we report the isolation, structure identification,and cytotoxic and antibacterial activity of cristatumin E (1).
|
Download:
|
| Fig. 1. The structures of cristatumin E (1) and homodimer (6) [13]. | |
Specific rotations were obtained on a JASCO P-1020 digital polarimeter. UV spectra were recorded on a Beckman DU 640 spectrophotometer. CD spectra were measured on a JASCO J-715 spectropolarimeter. IR spectra were taken on a Nicolet Nexus 470 spectrophotometer in KBr discs. NMR spectra were recorded on a JEOL JNM-ECP 600 spectrometer using TMS as the internal standard,and chemical shifts were recorded as δ values. ESIMS was utilized on a Q-TOF Ultima Global GAA076 LC mass spectrometer. Semipreparative HPLC was performed using an ODS column [YMC-pak ODS-A,10μm 250 mm,5 m,4μL/min]. Thin layer chromatography (TLC) and column chromatography (CC) were performed on plates precoated with silica gel GF254 (10- 40 μm) and over silica gel (200-300mesh,Qingdao Marine Chemical Factory),and a Sephadex LH-20 (Amersham Biosciences),respec- tively. Vacuum-liquid chromatography (VLC) was carried out over silica gel H (Qingdao Marine Chemical Factory). Sea salt used was made by the evaporation of sea water collected in Laizhou Bay (Weifang Haisheng Chemical Factory).
2.2. MaterialMarine fungus E. herbariorum HT-2 was isolated fromthe green marine alga E. prolifera that was collected from Qingdao’s first beach,Shandong province of China in June 2008. E. prolifera was identified by Dr. Tao Liu,College of Marine Life Science of Ocean University of China (OUC),and the specimen was deposited in Culture Collection of Seaweed at OUC. E. prolifera was cleaned by sterile water first and then soaked in alcohol for 5 seconds to disinfect the surface microbes,then washed again with sterile water. The tissue was mashed and dispersed on PDA plates (potatoes 200 g,glucose 20 g,agar 20 g,sea salt 33 g,chloramphenicol 0.1 g,pH 7.0,tap water 1 L) and incubated at 28 ℃ for 10 days. A single colony was transferred into another PDA plate and characterized as E. herbariorum according to its morphological characteristics and 18S rRNA gene sequences (GenBank accession No. JX839684). A reference culture is maintained in our laboratory at -80 ℃. The strainwas prepared on PDA agar slants and stored at 4 ℃.
2.3. Fermentation and extractionSpores were directly inoculated into a 1000 μL flask containing 300 μL fermentation media (mannitol 20 g,maltose 20 g,glucose 10 g,monosodium glutamate 10 g,yeast extract 3 g,KH2PO4 0.5 g, MgSO4 0.3 g,and sea salt 33 g dissolved in 1 L water,pH 6.5). The flasks were statically incubated at 25 ℃. After 30 days of cultivation,18 L of whole broth was extracted three times with EtOAc. The EtOAc extract was concentrated under reduced pressure to give a crude extract (48.8 g).
2.4. PurificationThe crude extract (48.8 g) was separated into 6 fractions on a silica gel column using step gradient elution with petroleum ether-CH2Cl2 (1:0-0:1) and then with CH2Cl2-MeOH (1:0-0:1). Fraction 4 (4.5 g) was further separated into 8 subfractions by Sephadex LH-20,eluting with MeOH. Subfraction 4-2 (630 mg) was then purified by semipreparative HPLC (50% MeOH-H2O, 4.0 μL/min) to yield 1 (45 mg,tR 14.1 min).
Cristatumin E (1): yellow amorphous powder; [α]D20 +232 (c 0.11,MeOH). UV (MeOH) λmax (logε): 204 (4.14),232 (3.91),292 (3.53) nm; CD (MeOH) λmax (Δε) 303 (+24.8),248 (+53.1) nm; IR (KBr,cm-1 ):υmax 3349,2963,2928,2867,1667,1462,1440,1347, 1313,1292,1252,1189,1143,1097,918,817,745; The data of 1H NMR (600 MHz,DMSO-d6 and CD3OD) and 13C NMR (150 MHz, DMSO-d6 and CD3OD) are listed in Table 1; HRESIMS m/z 569.2869 [M+H]+ (calcd. for C32H37N6O4,569.2871).
The 1H NMR and 13C NMR spectra,spectra of DEPT, 1H-1H COSY, HMQC,NOESY,HMBC,and IR of compound 1 can be found in Supporting information.
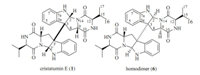
2.5. Determination of the absolute configuration by Marfey’ amino acid analysis [12]Compound 1 (1 mg) in 6 mol/L HCl (1 μL) was heated at 105 ℃ or 14 h. Then,the solutionwas evaporated and re-dissolved in H2O to 50 mmol/L. 50 μL of acid hydrolysates solution was placed in a 1.5 μL Eppendorf tube and treated with 1% solution of FDAA in acetone (200 μL) followed by 1.0 mol/L NaHCO3 (40 μL). The reaction mixture was heated at 45 ℃ for 1 h,cooled to room temperature,and then acidifiedwith 20 μL of 2.0 mol/L of HCl. In a similar fashion,FDAA derivatives of standard D- and DL-Val were prepared separately. The derivatives of the compound 1 and standard amino acids were subjected to HPLC analysis (YMC C18 column; 5 mm,4.6 mm × 250 mm; 1.0 mL/min; UV detection at λ 340 nm) at 30 ℃ using the following gradient program: solvent A, waterwith 1‰; solvent B,MeCN; linear gradient: 0 min 25% B, 40 min 60% B,45 min 100% B. The retention times for the FDAA derivatives of hydrolysates of 1,standard L-Val,and DL-Val are shown in Fig. 2.

|
Download:
|
| Fig. 2. HPLC profile for the FDAA derivatives of hydrolysates 1 (a),standard L-Val (b),standard DL-Val (c) and the mix derivatives of hydrolysates 1 and standard DL-Val (d). | |
| Table 1 1H NMR and 13C NMR data of 1 in DMSO-d6 and CD3OD (TMS,δ ppm). |
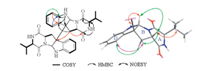
Compound 1 was obtained as a yellow amorphous powder. On the basis of HRESIMS,the molecular formula of 1 was assigned as C32H36N6O4,requiring 18 degrees of unsaturation. Only half of the proton and carbon signals were observed in 1H NMR and 13C NMR spectra of 1,indicating 1 is a symmetrical structure. Analysis of the 1H NMR, 13C NMR and DEPT NMR data for 1 (Table 1) and 2D NMR (Fig. 3) revealed that 1 has the same planar structure as homodimer 6 [13]. To determine whether compound 1 and homodimer 6 are the same compound,the 1H NMR and 13C NMR data were remeasured in CD3OD and the specific rotation was measured in the same solvent,CH3OH. The results showed that C-9 and C-10 of 1 were shifted upfield 0.5 and 1 ppm while C-8,C-12 and C-15-C-17 were shifted downfield for 1.1,2.3,3.6,1.8 and 0.5 ppm,respectively (Table 1). In addition,compound 1 showed different specific rotation ([α]D +232) with that of 6 ([α]D 273) [13],further suggesting that 1 as the stereoisomer of 6.
|
Download:
|
| Fig. 3. 1H-1H COSY,HMBC and NOESY correlations of 1. | |
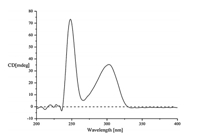
The relative configuration of 1 was identified by analyzing its NOESY data (Fig. 3). The NOESY correlations of H-9 (δH 4.11) with H-2 ((δH 4.89),H-8β ((δH 3.17),H-15 ((δH 1.94) and H-16 ((δH 0.79) suggested cis-fused hexahydropyrrolo[2,3-b]indole nucleus and cis-orientation of H-9,H-2 and 12-isopropyl group. The NOESY correlation between H-9 and H-4' ((δH 7.38) indicated anti- conformation around the C-3-C-3' bond. The absolute configuration of 1 was determined byMarfey’s amino acid analysis [12] and CD spectrum. The FDAA derivatives of hydrolysates of 1,standard L-Val and DL-Val were prepared and were analyzed by HPLC (Section 2). The retention time of the FDAA derivative of hydrolysates of 1 (tR 24.2 min) is the same to that of standard D-Val (tR 24.2 min) but different from that of standard L-Val (tR 20.0 min) (Fig. 2),suggesting the D-Val residue in 1. Furthermore, CD curve of 1 (Fig. 4) showed a positive Cotton effect at λmax 248 nm arose from the π-π* transitions of indoline ring. The positive and negative CD Cotton effects at λmax 248 nmcorrespond to the R- and S-configuration of C-3,respectively [13, 14, 15]. Thus,the absolute configuration of 1 was unambiguously established to be (2/2'R,3/3'R,9/9'S,12/12'R).When congested structure 6 converts into unfolded structure 1,the steric and shielding effects between the benzene ring and CH2-8,CH-12 and iPr-12 disappeared,and this resulted in the downfield shifts of C-8,C-12 and C-15-C-17. The upfield shift of C-10 in 1 could be explained by the shielding effect of the benzene ring on O=C-10.
|
Download:
|
| Fig. 4. The CD curve of 1. | |
Compound 1 was tested for antibacterial activity against E. aerogenes,Bacillus subtilis,Escherichia coli,Pseudomonas aeruginosa and Candida albican using the agar dilution method [16]. Compound 1 was also assayed for cytotoxicity against K562 and the MCF-7 cell line by the MTT method [17]. The details can be found in Supporting information. The results showed that compound 1 had weak antibacterial activity against B. aerogenes and E. coli,both with MIC values of 44 μmol/L (ciprofloxacin, positive control,MIC 0.47 and 0.24 μmol/L,respectively). Compound 1 displayed moderate cytotoxicity against K562 tumor cell line with an IC50 value of 8.3 μmol/L,while it was not cytotoxic to MCF-7 cell line (IC50 > 50 μmol/L) (adriamycin,positive control, IC50 1.73 and 2.5 μmol/L,respectively).
4. ConclusionCristatumin E (1) is a new cytotoxic bispyrrolidinoindoline diketopiperazine alkaloid with a homodimeric and bilaterally symmetric structure. In addition,cristatumin E (1) showed cytotoxicity against K562 tumor cell line and anti-bacterial activity against E. aerogenes and E. coli. Acknowledgments
This work is financially supported by NSFC (No. 21172204) & NSFS (No. ZR2009CQ014),863 Program of China (Nos. 2013AA092901,2012AA092104 and 2011AA09070106),973 Program of China (No. 2010CB833804),and Scientific Research Foundation for the Returned Overseas Chinese Scholars,Ministry of Education of China (No. [2010]1174).
Appendix A. Supplementary dataSupplementarymaterial related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.07.028.
| [1] | W.H. Gerwick, B.S. Moore, Lessons from the past and charting the future of marine natural products drug discovery and chemical biology, Chem. Biol. 19 (2012) 85- 98. |
| [2] | H. Greve, I.E. Mohamed, A. Pontius, et al., Fungal metabolites: structural diversity as incentive for anticancer drug development, Phytochem. Rev. 9 (2010) 537-545. |
| [3] | N. Benjamin, L.G. Kenneth, B.R. Miller, et al., Neuteboom STC (2006) NPI-2358 is a tubulin-depolymerization agent: in vitro evidence for activity as a tumor vascular- disrupting agent, Anticancer Drugs 17 (2006) 25-31. |
| [4] | (a) K. Kanoh, S. Kohno, T. Asari, et al., (-)-Phenylahistin: a new mammalian cell cycle inhibitor produced by Aspergillus ustus, Bioorg. Med. Chem. Lett. 7 (1997) 2847-2852;(b) K. Kanoh, S. Kohno, J. Katada, et al., (-)-Phenylahistin arrests cells in mitosis by inhibiting tubulin polymerization, J. Antibiot. 52 (1999) 134-141;(c) K. Kanoh, S. Kohno, J. Katada, et al., Antitumor activity of phenylahistin in vitro and in vivo, Biosci. Biotechnol. Biochem. 63 (1999) 1130-1133. |
| [5] | (a) J.K. Zheng, H.J. Zhu, K. Hong, et al., Novel cyclic hexapeptides from marinederived fungus, Aspergillus sclerotiorum PT06-1, Org. Lett. 11 (2009) 5262-5265;(b) Z.Y. Lu, Y. Wang, C.D. Miao, et al., Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102, J. Nat. Prod. 72 (2009) 1761- 1767;(c) Z.Y. Lu, H.J. Zhu, P. Fu, et al., Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum, J. Nat. Prod. 73 (2010) 911-914;(d) J.K. Zheng, Z.H. Xu, Y. Wang, et al., Cyclic tripeptides from the halotolerant fungus Aspergillus sclerotiorum PT06-1, J. Nat. Prod. 73 (2010) 1133-1137;(e) X.P. Peng, Y. Wang, K.L. Sun, et al., Cerebrosides and 2-pyridone alkaloids from the halotolerant fungus Penicillium chrysogenum grown in a hypersaline medium, J. Nat. Prod. 74 (2011) 1298-1302;(f) Y.B. Zhuang, X.C. Teng, Y. Wang, et al., New quinazolinone alkaloids within rare amino acid residue from coral-associated fungus, Aspergillus versicolor LCJ-5-4, Org. Lett. 13 (2011) 1130-1133;(g) Y.B. Zhuang, X.C. Teng, Y. Wang, et al., Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4, Tetrahedron 67 (2011) 7085-7089;(h) J.F. Wang, Z.Y. Lu, P.P. Liu, et al., Cytotoxic polyphenols from the fungus Penicillium expansum 091006 endogenous with mangrove plant Excoecaria agallocha, Planta Med. 78 (2012) 1861-1866;(i) J.F. Wang, P.P. Liu, Y. Wang, et al., Antimicrobial aromatic polyketides from gorgonian-associated fungus, Penicillium commune 518, Chin. J. Chem. 30 (2012) 1236-1242. |
| [6] | K.L. Sun, Y. Wang, P. Fu, P.P. Liu, W.M. Zhu, Studies on the secondary metabolites of Eurotium herbariorum HT-2 symbiotic with Enteromorpha prolifera, Chin. Mar. Drugs 32 (2013) 37-45. |
| [7] | J.R. Zhang, X.L. Tang, G.Q. Li, Studies on chemical constituents of Entermorpha prolifera, Period. Ocean Univ. China 40 (2010) 93-95. |
| [8] | Y. Okai, K.H. Okai, Potent anti-inflammatory activity of pheophytin a derived from edible green alga, Entermorpha prolifera (Sujiao-Nori), Int. J. Immunopharmacol. 19 (1997) 355-358. |
| [9] | G.J. Slack, E. Puniani, J.C. Frisvad, et al., Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities, Mycol. Res. 113 (2009) 480- 490. |
| [10] | V.D. Hauser, H.P. Weber, H.P. Sigg, Isolation and configuration of chaetocin, Helv. Chim. Acta 53 (1970) 1061-1073. |
| [11] | C.R. Isham, J.D. Tibodeau, A.R. Bossou, The anticancer effects of chaetocin are independent of programmed cell death and hypoxia, and are associated with inhibition of endothelial cell proliferation, Br. J. Cancer 106 (2012) 314-323. |
| [12] | P. Marfey, Determination of D-amino acids. II. Use of a bifunctional reagent 1,5- difluoro-2, 4-dinitrobenzene, Carlsberg Res. Commun. 49 (1984) 591-596. |
| [13] | C. Pérez-Balado, H. Sun, C. Griesinger, et al., Residual dipolar coupling enhanced NMR spectroscopy and chiroptics: a powerful combination for the complete elucidation of symmetrical small molecules, Chem. Eur. J. 17 (2011) 11983-11986. |
| [14] | C.J. Barrow, P. Cai, J.K. Snyder, et al., WIN 64821, a new competitive antagonist to substance P, isolated from an Aspergillus species: structure determination and solution conformation, J. Org. Chem. 58 (1993) 6016-6021. |
| [15] | C. Takahashi, K. Minoura, T. Yamada, et al., Potent cytotoxic metabolites from a Leptosphaeria species. Structure determination and conformational analysis, Tetrahedron 51 (1995) 3483-3498. |
| [16] | L.L. Zaika, Spices and herbs: their antimicrobial activity and its determination, J. Food Safety 9 (1988) 97-118. |
| [17] | T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, Immunol. Methods 65 (1983) 55-63. |