1. Introduction
In recent years,heteropolyacids have attractedmuch attention as environmentally benign catalysts for organic synthetic processes. They possess unique physicochemical properties,such as super-acidity,high thermal and chemical stability,ability to accept and release electrons,high proton mobility,and the possibility of varying their acidity and oxidizing potential [1]. Their significantly higher Brønsted acidity makes them more effective catalysts than conventional acid catalysts,such as mineral acids,ion exchange resins and mixed oxides zeolites [2]. They are non-corrosive,inexpensive and reusable and require less waste disposal [3]. They are used as industrial catalysts for several liquid phase reactions [4, 5, 6, 7].
Multicomponent reactions (MCRs),due to their operational simplicity,high bond forming efficiency,reduced waste and rapid access to structural diversity,have attracted much attention of synthetic organic chemists for building highly functionalized organic molecules and pharmacologically important heterocyclic compounds [8, 9, 10, 11]. Developing MCR protocols in aqueous medium is an active area of research in this direction. Water is recognized as an attractive medium for many organic reactions because it is the lowest cost,most abundantly available solvent. Water,as a green solvent,is highly polar and therefore immiscible with most organic compounds. Moreover,the water soluble catalyst resides and operates in the aqueous phase and separation of organic materials is thus easy. Also,dramatic rate enhancements have been achieved in water in many organic reactions [12, 13]. Reactions in aqueous media are generally environmentally safe,devoid of any carcinogenic effects,simple to handle,comparatively more economic and especially important in industry.
It is well known that fused pyran derivatives exhibit a wide spectrum of pharmacological activities and biological activities, such as insecticidal [14],antiviral and antileishmanial [15, 16], anticonvulsant and antimicrobial activities [17]. Also,many of them are non-peptide human immunodeficiency virus (HIV) protease inhibitors [18, 19, 20]. A literature survey revealed that comparatively few methods have been reported for the preparation of pyrano[4,3-b]pyran derivatives. Recently,a one-pot,threecomponent reaction of 4-hydroxy-6-methylpyran-2-one with malononitrile and aromatic aldehydes has enjoyed wider utilization in the synthesis of these compounds. A variety of reagents,such as piperidine [21, 22],triethylbenzyl ammonium chloride [23],KF/Al2O3 [24],magnesium oxide [25],ionic liquids [16, 26],DBU [27],and also without any catalyst [28] have been employed to accomplish this transformation. Some of the reported methods suffer from serious limitations,such as long reaction times,complex synthetic pathways,tedious work-up, use of organic solvents,lower product yields and non-reusability of the catalyst. Therefore,it is still necessary to develop clean, efficient and convenient methods to construct such significant heterocyclic compounds.
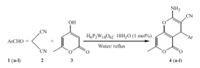
Based on the previous studies on the use of heteropolyacids as catalysts,and in a continuation of our endeavors for the development of simple and highly expedient methods for the synthesis of polyfunctionalized heterocycles of biological importance [29],we examined the possibility of using H6P2W18O62·18H2O,a Wells- Dawson type heteropolyacid,as a catalyst for the one-pot synthesis of pyrano[4,3-b]pyran derivatives by condensing aromatic aldehydes, malononitrile and 4-hydroxy-6-methylpyran-2-one in water under reflux (Scheme 1).
|
Download:
|
| Scheme 1.. H6P2W18O62·18H2O catalyzed synthesis of 2-amino-4-aryl-3-cyano-5- oxo-4H,5H-pyrano[4,3-b]pyrans in water. . | |
2. Experimental
All the chemicals were purchased from Merck and Sigma- Aldrich and used without further purification. Melting points were determined in open glass capillaries and are uncorrected. IR spectra were recorded on a Perkin Elmer-1430 spectrophotometer using potassium bromide discs. 1H NMR and 13C NMR spectra were obtained at 400 MHz with a Bruker WM-400 spectrometer using DMSO-d6 as solvent and TMS as an internal standard. MS spectra were measured at Micromass ZMD ESI (70 eV) system. 2.1. General procedure for the synthesis of 2-amino-4-aryl-5-oxo-4H, 5H-pyrano[4,3-b]pyran-3-carbonitriles
A mixture of 4-hydroxy-6-methylpyran-2-one (1 mmol),aromatic aldehyde (1 mmol),malononitrile (1.2 mmol) and H6P2W18O62·18H2O (1 mol%) in 20 mL of water was stirred under reflux for appropriate time. After completion of the reaction as monitored by TLC,the reaction mixture was cooled to room temperature. The solid product was collected by filtration,washed with aqueous ethanol (1:1) and recrystallized from ethanol to yield the pure product. All products were characterized by their spectral and physical data. 2.2. Characterization data for the representative compounds 2.2.1. 2-Amino-7-methyl-5-oxo-4-phenyl-4H,5H-pyrano[4,3-b]pyran -3-carbonitrile (4a,C16H12N2O3)
Yellow crystals; yield: 92%; Mp 234-236 ℃ (236-238 ℃) [22]; IR (KBr,cm-1): ν 3458,3260,3131,3088,2293,1649,1555,1342, 1053,790; 1H NMR (400 MHz,DMSO-d6): δ 2.56 (s,3H,CH3),4.76 (s,1H,CH),6.43 (s,1H==CH),7.20-7.22 (dd,2H,ArH,Ja = 3.9 Hz, Jb = 1 Hz),7.27 (s,2H,NH2),7.42 (d,1H,ArH,J = 3.5 Hz),7.63-7.65 (dd,2H,ArH,Ja = 4.2 Hz,Jb = 0.9 Hz); 13C NMR (100 MHz,DMSOd6): δ 23.15,31.94,57.84,103.97,116.22,118.94,122.51,124.27, 124.58,124.67,127.72,140.90,152.95,153.84,158.40,159.49. 2.2.2. 2-Amino-4-(4-methoxyphenyl)-7-methyl-5-oxo-4H,5Hpyrano[ 4,3-b]pyran-3-carbonitrile (4h,C17H14N2O4) Yellow crystals; yield: 86%; Mp 202-205 ℃ (205-207 ℃) [22]; IR (KBr,cm-1): ν 3472,3365,2925,2212,1637,1540,1296,1039, 824; 1H NMR (400 MHz,DMSO-d6): δ 2.57 (s,3H,CH3),3.76 (s,3H, OCH3),5.13 (s,1H,CH),6.35 (s,1H==CH),6.65-6.66 (m,2H,ArH); 7.41 (m,2H,ArH),7.57 (s,2H,NH2); 13CNMR(100 MHz,DMSO-d6):
δ 23.27,30.49,55.87,101.64,111.55,112.90,116.17,118.86, 122.44,124.11,150.92,151.67,152.05,153.94,158.71,159.50. 2.2.3. 2-Amino-4-(furan-2-yl)-7-methyl-5-oxo-4H,5H-pyrano[4,3- b]pyran-3-carbonitrile (4i,C14H10N2O4)
Pink crystals; yield: 95%; Mp 223-225 ℃ (223-224 ℃) [29]; IR (KBr,cm-1): ν 3208,3085,2195,1620,1384,1258,1139,1013, 980,754; 1H NMR (400 MHz,DMSO-d6): δ 2.25 (s,3H,CH3),4.47 (s, 1H,CH),6.10 (s,1H==CH),6.16 (d,1H,ArH,J = 3.1 Hz); 6.30 (dd, 1H,ArH,Ja = 1.8,Jb = 1.2 Hz); 6.97 (s,2H,NH2),7.37 (d,1H,ArH, J = 1.0 Hz); 13C NMR (100 MHz,DMSO-d6): δ 19.43,29.88,55.36, 97.95,98.40,105.90,110.17,118.89,141.55,154.12,158.58, 158.75,161.13,162.36. MS (m/z): 270.0 (M+). 2.2.4. 2-Amino-7-methyl-5-oxo-4-(thiophen-2-yl)-4H,5Hpyrano[ 4,3-b]pyran-3-carbonitrile (4j,C14H10N2O3S)
Colorless crystals; yield: 92%; Mp 242-244 ℃ (242-244 ℃) [29]; IR (KBr,cm-1): ν 3081,2857,2196,1717,1614,1375,1254, 1189,1044,777; 1H NMR (400 MHz,DMSO-d6): δ 2.23 (s,3H,CH3), 4.66 (s,1H,CH),6.13 (s,1H==CH),6.91 (dd,1H,ArH,Ja= 3.5 Hz, Jb = 1.5 Hz); 6.97 (d,1H,ArH,J = 2.9 Hz); 7.13 (s,2H,NH2),7.25 (d, 1H,ArH,J = 3.9 Hz); 13C NMR (100 MHz,DMSO-d6): δ 19.40,31.26, 57.81,97.94,100.89,119.03,124.44,124.63,126.61,147.78, 157.72,158.44,161.22,162.57. MS (m/z): 286.1 (M+). 2.2.5. 2-Amino-7-methyl-4-(5-methyl-thiophen-2-yl)-5-oxo-4H,5Hpyrano[4,3-b]pyran-3-carbonitrile (4k,C15H12N2O3S)
Brown crystals; yield: 93%; Mp 176-177 ℃ (175-177 ℃) [29]; IR (KBr,cm-1): ν 3471,3363,3119,2923,2211,1639,1549,1296, 1039,743; 1H NMR (400 MHz,DMSO-d6): d 2.24 (s,3H,CH3),2.39 (s,3H,CH3),4.57 (s,1H==CH),6.04 (s,1H==CH),6.55 (d,1H,ArH, J = 1.6 Hz),6.74 (d,1H,ArH,J = 3.3 Hz),6.84 (s,2H,NH2); 13C NMR (100 MHz,DMSO-d6): δ 14.97,19.44,31.30,58.19,97.89,101.02, 118.97,124.29,124.47,138.17,140.64,157.51,158.28,161.30, 162.10. MS (m/z): 301.1 (M++1). 2.2.6. 2-Amino-4-(5-chlorothiophen-2-yl)-7-methyl-5-oxo-4H,5Hpyrano[ 4,3-b]pyran-3-carbonitrile (4l,C14H9ClN2O3S)
Yellow crystals; yield: 89%; Mp 226-227 ℃ (228-229 ℃) [29]; IR (KBr,cm-1): ν 3322,3191,3112,2196,1671,1606,1514,1344, 1262,1037,732; 1H NMR (400 MHz,DMSO-d6): d 2.22 (s,3H,CH3), 4.24 (s,1H,CH),6.13 (s,1H==CH),6.84 (d,1H,ArH,J = 2.7 Hz),6.93 (s,2H,NH2),7.11 (d,1H,ArH,J = 1.5 Hz); 13C NMR (100 MHz, DMSO-d6): δ 19.35,35.47,58.28,97.89,101.18,119.19,123.95, 128.44,133.26,135.34,159.97,161.28,162.07,164.28. MS (m/z): 320.0 (M+). 3. Results and discussion We report herein a green approach for the synthesis of pyrano[4,3-b]pyrans catalyzed by H6P2W18O62·18H2O in water under reflux. In our initial study,the reaction of 4-hydroxy-6- methylpyran-2-one,benzaldehyde and malononitrile was used as a model reaction to optimize the reaction conditions. The model reaction was carried out in the presence of a variety of catalysts and solvents under different conditions. The results obtained are summarized in Table 1 and determined that the best results in terms of reaction time,cost and yield were obtained with 1 mol% of H6P2W18O62·18H2O as catalyst in water under reflux. Higher loading of the catalyst did not improve the product yield to a great extent (Table 1,entry 9). The product formation was also observed under reaction conditions at room temperature. However,the yield was unsatisfactory (62%) and the reaction was incomplete even after 4 h (Table 1,entry 8). Thus,refluxing all the components in presence of 1 mol% of H6P2W18O62·18H2O in water proved to be the optimum conditions for this reaction.
| Table 1 Optimization of the reaction conditions for the synthesis of 4a. |
Using these optimized reaction conditions,a series of pyrano[ 4,3-b]pyran derivatives (4a-l) were prepared in high to excellent yields from different aromatic aldehydes having electron- donating as well as electron-withdrawing groups. These results are listed in Table 2. It is noteworthy to mention that the methodology worked well for heteroaromatic aldehydes (Table 2, entries 9-12).
| Table 2 Synthesis of 2-amino-4-aryl-3-cyano-5-oxo-4H,5H-pyrano[4,3-b]pyrans in water using H6P2W18O62·18H2O (1 mol%) as catalyst under reflux. |
A plausible mechanism for the above reaction is depicted in Scheme 2. The reaction may proceed via an in situ initial formation of the arylidenenitrile from the Knoevenagel condensation of an aldehyde with malononitrile. The unsaturated nitrile then undergoes subsequent reactions with 4-hydroxy-6-methylpyran- 2-one to give the final product.
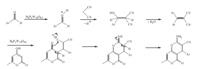
|
Download:
|
| Scheme 2..Probable reaction pathway for the formation of pyrano[4,3-b]pyrans. | |
Recovery and reuse of the catalyst and reaction medium was investigated by using the model reaction. After the reaction was completed,the product was collected by simple filtration and the aqueous filtrate containing H6P2W18O62·18H2O was used as such for the next reaction run. Again,the product 4a was obtained in comparative yield. Following four consecutive reaction cycles, there was a slight decrease in yield (94%,92%,87% and 84%). Since the solvent containing catalyst is reused ‘as is’,we believe that any organic impurities generated in each run may cause the decrease in yield observed after each use.
Furthermore,to show the advantages of this methodology in comparison with previously reported procedures,we selected the synthesis of 4a as a representative example. As can be seen from Table 3,the reaction catalyzed by H6P2W18O62·18H2O in water gave a comparable yield and requires less time than other protocols.
| Table 3 Comparison of the present method with other reported protocols for the synthesis of 4a. |
In conclusion,we have developed a green,efficient and convenient approach for the synthesis of pyrano[4,3-b]pyran derivatives using H6P2W18O62·18H2O as a catalyst in water. Reusability of the catalyst and reaction media,use of a non-toxic and inexpensive catalyst with operational simplicity makes this method an attractive choice for the preparation of pyrano[4,3-b]pyrans. Acknowledgment
We thank the Director,SAIF,Panjab University,Chandigarh for NMR and MS spectral data. We are also grateful to Dr. Aman Deep Acharya for his valuable scientific suggestions.
| [1] | S. Allameh, M.M. Heravi, M.M. Hashemi, et al., Synthesis of 3-(aryl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one derivatives using heteropolyacids as green, heterogeneous and recyclable catalysts, Chin. Chem. Lett. 22 (2011) 131. |
| [2] | S. Allameh, M.M. Hashemi, M.M. Heravi, et al., Synthesis of quinazolin-4(3H)-one derivatives using heteropolyacids as heterogeneous and recyclable catalysts, Asian J. Chem. 23 (2011) 1588. |
| [3] | M.H. Alizadeh, H. Razavi, F.F. Bamoharram, et al., The oxidative cleavage of carbon tin bond catalyzed by heteropolyacids ofmolybdenum, J. Mol. Catal. 206 (2003) 89. |
| [4] | I.V. Kozhevinikov, Heteropoly acids and related compounds as catalysts for fine chemical synthesis, Catal. Rev. Sci. Eng. 37 (1995) 311. |
| [5] | Y. Izumi, R. Hasebe, K. Urabe, Catalysis by heterogeneous supported heteropoly acid, J. Catal. 84 (1983) 402. |
| [6] | H. Soeda, T. Okuhara, M. Misono, Selective alkylation of p-xylene with 2-methylpropene by 12-tungstophosphoric acid, Chem. Lett. 23 (1994) 909. |
| [7] | M.M. Heravi, B.A. Jani, F. Derikvand, F.F. Bamoharram, H.A. Oskooie, Three component, one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives in the presence of H6P2W18O62 18H2O as a green and recyclable catalyst, Catal. Commun. 10 (2008) 272. |
| [8] | C.O. Kappe, Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog, Acc. Chem. Res. 33 (2000) 879. |
| [9] | F. Liu, T. Evans, B.C. Das, Synthesis of 2-substituted 2H-chromenes using potassium vinyltrifluoroborates, Tetrahedron Lett. 49 (2008) 1578. |
| [10] | J. Zhu, H. Bienayme, Multicomponent Reactions, 1st ed., Wiley-VCH, Weinheim, 2005. |
| [11] | A. Domling, I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem. Int. Ed. 39 (2000) 3168. |
| [12] | C.J. Li, Organic reactions in aqueous media-with a focus on carbon-carbon bond formation, Chem. Rev. 93 (1993) 2023. |
| [13] | A. Meijer, S. Otto, J.B.F.N. Engberts, Effects of the hydrophobicity of the reactants on Diels-Alder reactions in water, J. Org. Chem. 63 (1998) 8989. |
| [14] | M. Uher, V. Konecny, O. Rajniakove, Synthesis of 5-hydroxy-2-hydroxymethyl-4H-pyran-4-one derivatives with pesticide activity, Chem. Pap. 48 (1994) 282. |
| [15] | M. Perez-Perez, J. Balzarini, J. Rozenski, et al., Synthesis and antiviral activity of phosphonate derivatives of enantiomeric dihydro-2H-pyranyl nucleosides, Bioorg. Med. Chem. Lett. 5 (1995) 1115. |
| [16] | X. Fan, D. Feng, Y. Qu, et al., Practical and efficient synthesis of pyrano[3,2-c]pyridone, pyrano[4,3-b]pyran and their hybrids with nucleoside as potential antiviral and antileishmanial agents, Bioorg. Med. Chem. Lett. 20 (2010) 809. |
| [17] | M.D. Aytemir, U. Calis, M. Ozalp, Synthesis and evaluation of anticonvulsant and antimicrobial activities of 3-hydroxy-6-methyl-2-substituted 4H-pyran-4-one derivatives, Arch. Pharm. 337 (2004) 281. |
| [18] | S. Wang, G.W.A. Milne, X. Yang, et al., Discovery of novel, non-peptide HⅣ-1 protease inhibitors by pharmacophore searching, J. Med. Chem. 39 (1996) 2047. |
| [19] | L. Pochet, C. Doucet, M. Schynts, et al., Esters and amides of 6-(chloromethyl)-2-oxo-2H-1-benzopyran-3-carboxylic acid as inhibitors of a-chymotrypsin: significance of the "aromatic" nature of the novel ester-type coumarin for strong inhibitory activity, J. Med. Chem. 39 (1996) 2579. |
| [20] | A. Mazumder, S. Wang, N. Neamati, et al., Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease, J. Med. Chem. 39 (1996) 2472. |
| [21] | M.Z. Piao, K. Imafuku, Convenient synthesis of amino-substituted pyranopyranones, Tetrahedron Lett. 38 (1997) 5301. |
| [22] | E.V. Stoyanov, I.C. Ivanov, D. Heber, General method for the preparation of substituted 2-amino-4H,5H-pyrano[4,3-b]pyran-5-ones and 2-amino-4H-pyrano[3,2-c]pyridine-5-ones, Molecules 5 (2000) 19. |
| [23] | D.Q. Shi, L.H. Niu, Q.Y. Zhuhang, One-pot three-component synthesis of pyrano[3,2-c]pyran-5-one derivatives in aqueous medium, Chin. J. Org. Chem. 28 (2008) 1633. |
| [24] | X.S. Wang, J.X. Zhou, Z.S. Zeng, et al., One-pot synthesis of pyrano[3,2-c]pyran derivatives catalyzed by KF/Al2O3, Arkivoc 11 (2006) 107. |
| [25] | M. Seifi, H. Sheibani, High surface areaMgOas a highly effective heterogeneous base catalyst for three-component synthesis of tetrahydrobenzopyran and 3,4-dihydropyrano[c]chromene derivatives in aqueous media, Catal. Lett. 126 (2008) 275. |
| [26] | A. Shaabani, S. Samadi, Z. Badri, et al., Ionic liquid promoted efficient and rapid onepot synthesis of pyran annulated heterocyclic systems, Catal. Lett. 104 (2005) 1. |
| [27] | J.M. Khurana, B. Nand, P. Saluja, DBU: a highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano[4,3-b]pyranes, 2-amino-4H-benzo[h]chromenes and 2-amino-4H-benzo[g]-chromenes in aqueous medium, Tetrahedron 66 (2010) 5637. |
| [28] | A. Shaabani, S. Samadi, A. Rahmati, One-pot, three-component condensation reaction in water: an efficient and improved procedure for the synthesis of pyran annulated heterocyclic systems, Synth. Commun. 37 (2007) 491. |
| [29] | D. Rajguru, B.S. Keshwal, S. Jain, Solvent-free, green and efficient synthesis of pyrano[4,3-b]pyrans by grinding and their biological evaluation as antitumor and antioxidant agents, Med. Chem. Res. (2013), http://dx.doi.org/10.1007/s00044-013-0586-4. |