1. Introduction
Flavanoids are a unique class of naturally occurring products existing in the plant kingdom,and exhibiting a wide range of interesting physiological properties,such as in hypotensive [1], antifungal [2],antibacterial [3, 4],and antitumor [5] areas. This wide range of biological properties has stimulated interest in the synthesis of such naturally occurring flavanoids.
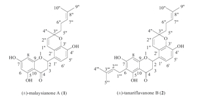
Two naturally occurring flavanoid compounds,(±)-malaysianone A (1) and(±)-tanariflavanones B (2) (Fig. 1),had been isolated fromthe genus Macaranga. The genus Macaranga as one of the largest genera of the Euphorbiaceae family has approximately 300 species [6]. Malaysianone A was isolated from the inflorescences of Macaranga triloba [7]. M. triloba are widely distributed in tropical and subtropical regions and used as folkmedicines [8]. Tanariflavanones Bwas isolated from the fallen leaves of Macaranga tanarius and showed inhibition of radicle growth of lettuce seedlings at 200 ppm [9]. As far as we know, the total synthesis of 1 and 2 has not been reported.
|
Download:
|
| Fig. 1.The structures of compounds 1 and 2. | |
In continuation of our ongoing program on the studies of flavanoids in our laboratory [10, 11, 12, 13, 14],we report herein a facile synthetic approach (Scheme 1) for the synthesis of compound (±)- malaysianone A and (±)-tanariflavanones B.
|
Download:
|
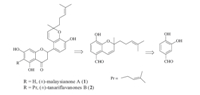
| Scheme 1.. Retrosynthetic analysis of (±)-malaysianone A (1) and (±)-tanariflavanones B (2). . | |
2. Experimental
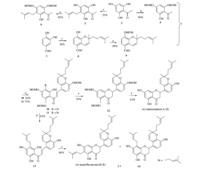
General experimental procedures: Melting points were determined on a X-5 Melting Point apparatus and are uncorrected. For column chromatography and TCL was performed on 200-300 mesh silica gel and GF254,respectively. 1H NMR and 13C NMR spectra were recorded in CDCl3 on a Bruker AM-400 MHz spectrometer using TMS as an internal standard at 400 MHz and 100 MHz,respectively. Mass spectral measurements were carried out on a Thermo Scientific LTQ-Orbitrap-Discovery (Bremen, Germany) spectrometer. 3. Results and discussion (±)-Malaysianone A (1) and (±)-tanariflavanones B (2) both possess a similar B-ring configuration and can be prepared from compound 9 and the corresponding acetophenones 4 and 6 (Scheme 1). The total synthesis of 1 and 2 is shown in Scheme 2. The key step was the synthesis of 5-formaldehyde-8-hydroxy-2-[40-methyl-30- penteneyl]-dihydro-1-benzopyran (8). The key intermediate 8 was synthesized from compound 7 and citral in anhydrous pyridine to 21% yield by using a known method [15]. It is noteworthy that we have modified the method reported by Jaipetch et al. to improve the yield of compound 8 from 21% to 30% by simultaneous addition of the dehydrating agent (i.e.,Na2SO4,MgSO4) in the reaction system. Protection of compound 8 by methoxymethylation (chloromethyl methyl ether: MOMCl,potassium carbonate) provided the crucial intermediate 9 in 89% yield. Compound 4 prepared from 2,4,6- trihydroxyacetophenone condensed with compound 9 in hydrous ethanolic solution by the action of potassium hydroxide-water- ethanol under nitrogen to give chalcone 10 in 82% yield. The subsequent cyclization was carried out with NaOAc in refluxing EtOH for 24 h to give 12 in 95% yield,followed by hydrolysis with 3 mol/L HCl in methanol to afford 1 in 62% yield. The synthetic product has identical spectral data with those of the natural product [7].
|
Download:
|
| Scheme 2.. Conditions and reagents: (a) prenyl bromide,anhydrous K2CO3,THF; (b) anhydrous K2CO3,acetone,MOMCl,reflux; (c) citral,anhydrous pyridine,reflux; (d) KOHH2O-EtOH,N2,reflux; (e) EtOH,NaOAc,reflux; (f) MeOH,HCl,reflux; (g) Dowex 50X2 resin,MeOH,reflux. . | |
Treatment of 2,4,6-trihydroxyacetophenone (3) and prenyl bromide with potassium carbonate in refluxing THF for 6 h gave compound 5 in 35% yield. Standard methoxymethylation (chloromethyl methyl ether:MOMCl,potassium carbonate) of 5 provided compound 6 in 85% yield. To a stirred mixture of KOH in H2O-EtOH was added compound 6 and compound 9 in EtOH cooled to 0 ℃ under nitrogen to give the chalcone 11 in 75% yield.
In our early study,we have reported the selective synthesis of C- 6 and C-8 prenylated flavanoids from chalcone [14]. Direction cyclization of chalcone 11 will not yield C-6 flavanoids,but result in C-8 prenylated flavanoids. The (±)-tanariflavanones B (2) was synthesized as an isomeric mixture in 52% yield from chalcone 11 by a two-step sequence: (1) demethoxymethylation and (2) cyclization. Demethoxymethylation of chalcone 11 by using classical reagents, such as p-toluenesulfonic acid and hydrochloric acid (3 mol/L HCl in methanol) for demethoxymethylation all resulted not only in poor yield of the desired product,but also gave more by-products and an intractable mixture. Deprotection of chalcone 11 with Dowex 50X2 resin afforded 13 in 65% yield. Dowex 50X2 resin is a favorable reagent previously tested for the deprotection of chalcone 11. Cyclization of chalcone 13 by treatment with NaOAc in EtOH gave the final compounds 2 and 14 in a ratio of 2:1 in 80% yield. The spectroscopic data of synthetic product was agreement with those of the natural products [9]. 4. Conclusion
In summary,this paper described a concise and efficient synthetic route for the first reported total synthesis of (±)- malaysianone A (1) and (±)-tanariflavanones B (2). The spectroscopic data of synthetic materials 1 and 2 were in agreement with those reported in the literature [16]. Not only does this research on the total synthesis of 1 and 2 have theoretical importance,but also has potential medical prospects.
Acknowledgment
This work was financially supported by the National Natural Science Foundation (Nos. 21162021,20962016),and the Program for New Century Excellent Talents in University (No. NCET-09- 0860).
| [1] | S. Ghatta, D. Nimmagadda, X. Xu, et al., Large-conductance, calcium-activated potassium channels: structural and functional implications, Pharmacol. Ther. 110 (2006) 103-116. |
| [2] | L. Toro, M. Wallner, P. Meera, et al., Maxi-K(Ca), a unique member of the voltagegated K channel superfamily, News Physiol. Sci. 13 (1998) 112-117. |
| [3] | T.R. Seshadri, Recent developments in the chemistry of flavonoids, Tetrahedron 6 (1959) 169-200. |
| [4] | T.L. Meragelman, K.D. Tucker, T.G. McClord, et al., Antifungal flavonoids from Hildegardia barteri, J. Nat. Prod. 68 (2005) 1790-1792. |
| [5] | M.J. Salvatore, A.B. Kng, A.C. Graham, et al., Antibacterial activity of lonchocarpol A, J. Nat. Prod. 61 (1998) 640-642. |
| [6] | M.M. Rahman, A.I. Gray, Antibacterial and antifungal activities of the constituents of Flemingia paniculata, P. Hkondkar, Pharm. Biol. 46 (2008) 356-359. |
| [7] | K.V. Hirpara, P. Aggarwal, A.J. Mukherjee, et al., Quercetin and its derivatives: synthesis, pharmacological useswith special emphasis on antitumor properties and prodrug with enhanced bioavailability, Curr. Med. Chem. 9 (2009) 138-161. |
| [8] | G. Webster, Synthesis of the genera and suprageneric taxa of Euphorbiaceae, Ann. Missouri Bot. Gard. 81 (1994) 33-144. |
| [9] | I. Zakaria, N. Ahmat, F.M. Jaafar, et al., Flavonoids with antiplasmodial and cytotoxic activities of Macaranga triloba, Fitoterapia 83 (2012) 968-972. |
| [10] | E.J.H. Corner, Wayside Trees of Malaya, 2th ed., United Selangor Press, Kuala Lumpur, 1998. |
| [11] | M.H. Tseng, C.H. Chou, Y.M. Chen, et al., Allelopathic prenylflavanones from the fallen leaves of Macaranga tanarius, J. Nat. Prod. 64 (2001) 827-828. |
| [12] | J.H. Yang, J.S. Luo, D.D. Guo, et al., Total synthesis of (±)-5,30-dihydroxy-40-methoxy-600-dimethyl-chromeno-(7,8,200,300)-flavanone, Chin. J. Org. Chem. 32 (2012) 1749-1752. |
| [13] | J.H. Yang, W.B. Zuo, D.D. Guo, et al., First total synthesis of four natural prenylated flavonoids, Chin. Chem. Lett. 23 (2012) 1375-1377. |
| [14] | J.H. Yang, W.Q. Huang, J.S. Luo, et al., First total synthesis of (±)-sepicanin A, Chin. Chem. Lett. 23 (2012) 127-129. |
| [15] | W.B. Zuo, J.H. Yang, H.J. Li, et al., Study on total synthesis of four natural prenylated flavonoids, Chin. J. Org. Chem. 32 (2012) 2276-2282. |
| [16] | J.H. Yang, Y.H. Zhang, H.J. Li, et al., First total synthesis of (±)-puyanin and (±)-40-O-methylbonannione, Chin. Chem. Lett. 21 (2010) 1267-1269. |
| [17] | T. Jaipetch, S. Kanghae, O. Pancharoen, et al., Constituents of Boesenbergia pandurata (syn Kaempferia pandurata): isolation, crystal structure and synthesis of (±)-boesenbergin A, Aust. J. Chem. 25 (1982) 351-361. |
| [18] | Spectral data of (±)-malaysianone A: White solid, mp 89-91 ℃; 1H NMR (400 MHz, CDCl3): δ 12.06 (s, 1H, OH-5), 6.91 (dd, 1H, J = 1.6 Hz, J = 8.4 Hz, H-50), 6.85 (d, 1H, J = 8.0 Hz, H-60), 6.61 (d, 1H, J = 10 Hz, H-200), 6.18 (s, 1H, OH-40), 6.01 (d, 1H, J = 2.4 Hz, H-6), 5.98 (d, 1H, J = 2.4 Hz, H-8), 5.69 (d, 1H, J = 10.4 Hz, H-100), 5.60 (s, 1H, OH-7), 5.53 (dd, 1H, J = 2.8 Hz, J = 13.2 Hz, H-2), 5.08 (dt, 1H, J = 1.2 Hz, J = 5.6 Hz, H-700), 3.15 (dd, 1H, J = 13.2 Hz, J = 17.2 Hz, H-3ax), 2.75 (dd, 1H, J = 3.2 Hz, J = 17.2 Hz, H-3eq), 2.10-2.06 (m, 2H, H-600), 1.79-1.68 (m, 2H, H-500), 1.67, 1.57 (s, 6H, 2×CH3, H-900 and 1000), 1.43 (d, 3H, J = 6.0 Hz, CH3, H-400); 13C-NMR (100 MHz, CDCl3,): δ 196.4 (s, C-4), 164.9 (s, C-7), 164.2 (s, C-5), 163.3 (s, C-9), 145.0 (s, C-40), 139.6 (s, C-30), 132.1 (s, C-800), 131.0 (s, C-200), 124.6 (s, C-10), 123.6 (s, C-700), 118.9 (s, C-20), 118.8(d, C-60), 118.6 (s, C-100), 114.5 (s, C-50), 103.0 (s, C-10), 96.8 (s, C-8), 95.6 (s, C-6), 79.0 (s, C-300), 76.0 (d, C-2), 42.3 (s, C-3), 40.7 (s, C-500), 26.0 (d, C-400), 25.6 (s, C-1000), 22.7 (s, C-600), 17.6 (s, C-900). HREIMS (m/z): 423.18039 [M+H]+ (calcd. for C25H26O6: 423.18022). Spectral data of (±)-tanariflavanones B (C30H34O6): Brownish oil, 1HNMR (400 MHz, CDCl3,): d 12.4 (s, 1H, OH-5), 6.90 (d, 1H, J = 8.4 Hz, H-60), 6.84 (d, 1H, J = 8.4 Hz, H-50), 6.60 (d, 1H, J = 10 Hz, H-100), 6.33 (s, 1H, OH-40), 5.99 (s, 1H, H-8), 5.68 (d, 1H, J = 10 Hz, H-200), 5.60 (s, 1H, OH-7), 5.50 (dd, 1H, J = 13.6 Hz, J = 2.8 Hz, H-2), 5.24 (dt, 1H, H-2000), 5.08 (dt, 1H, H-700), 3.35 (d, 2H, J = 7.2 Hz, H-1000), 3.16 (dd, 1H, J = 13.2 Hz, J = 17.2 Hz, H-3ax), 2.75 (dd, 1H, J = 2.8 Hz, J = 17.2 Hz, H-3eq), 2.10-2.12 (m, 2H, CH2, H-600), 1.82-1.77 (m, 2H, H-500), 1.57, 1.67, 1.72, 1.75 (s, 12H, 4CH3), 1.42 (d, 3H, CH3); 13CNMR(100 MHz, CDCl3,): d 196.3 (s, C-4), 163.7 (s, C-7), 161.3 (s, C-9), 161.1 (s, C-5), 145.1 (s, C-40), 139.6 (s, C-30), 135.5 (s, C-3000), 132.1 (s, C-800), 130.9 (d, C-200), 124.8 (d, C-700), 123.6 (s, C-10), 121.5 (d, C-2000), 118.9 (d, C-100), 118.7 (d, C-60), 118.6 (s, C-20), 114.5 (d, C-50), 107.1 (s, C-6), 102.9 (s, C-10), 95.5 (d, C-8), 79.0 (s, C-300), 76.0 (d, C-2), 42.5 (d, C-3), 40.7 (s, C-500), 26.1 (d, C-400), 25.8 (s, C-4000), 25.6 (s, C-1000), 22.7 (s, C-600), 21.1 (s, C-1000), 17.8 (d, C-5000), 17.6 (s, C-900). HREIMS (m/z): 491.2440 [M+H]+. |




