1. Introduction
Highly selective transformations,capable of differentiating similarly reactive groups in the same molecule,are desirable in synthetic chemistry [1]. Tolylene-2,4-diisocyanate (2,4-TDI) is an important asymmetrical diisocyanate. Its isocyanate groups can react with diols or diamines to produce polyurethanes or polyureas. They are widely used in many areas such as foamed plastics,structural elastomers,coating elastomers,adhesives, leather-like materials and auxiliary agents [2]. Although the reactivity of the two isocyanate groups in 2,4-TDI is obviously different [3],it is still very hard to achieve selectivity under common conditions. For example,the reaction kinetics of 2,4- TDI and methanol has been reported to be a parallel and consecutive reaction containing two simultaneous reaction paths [4].
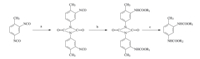
In order to study the selective reaction of diisocyanate with regard to carbamate or carbamide formation,different synthetic methodologies with isocyanate blocking have been reported [5]. Blocking agents,also known as protecting agents,are mainly phenols [6],oximes [7],uretdiones [8],imidazoles and secondary amines [9],among which uretdiones are the only molecules that distinguish different positions of 2,4-TDI. In our previous work [10],several asymmetrically substituted dicarbamates were synthesized through the uretdione route (Fig. 1). It was a convenient and economical route. However,the trimerization of 2,4-TDI occured easily [11],which was an irreversible side reaction and lowered the yield of product. In this paper,cyclohexanone oxime is used as a protecting agent to block the isocyanate. The reactivity of otho- and para- isocyanate groups in 2,4-TDI appears very distinct from each other and they react selectively with amino groups. Moreover,under controlled conditions,oxime-blocked isocyanate can only react with the -NH2 group of ethanolamine containing both the -OH and -NH2 groups.
|
Download:
|
| Fig. 1.The uretdione route to synthesis of asymmetric substituted dicarbamate: (a) (t-Bu)3P,toluene,0 ℃,3 h; (b) R1OH,chlorobenzene, 60 ℃,3 h; (c) R2OH,(t-Bu)3P,DMF, 90 ℃,3 h. | |
Cyclohexanone oxime (6.78 g,60.0 mmol) was dissolved in toluene (30.0 mL) under magnetic stirring,then tolylene-2,4- diisocyanate (5.05 g,29.0 mmol) was rapidly added and the reaction mixture was allowed to stir at room temperature for 1 h. After that,toluene was evaporated from the reaction system and a white solid was obtained. The product was washed with water three times to remove the excessive cyclohexanone oxime, and the precipitate was dried in vacuo. Yield: 92%. 1H NMR (400 MHz,DMSO-d6): δ 9.543 (s,1H,-NH-),8.892 (s,1H,-NH-), 7.570 (s,1H,Ph-H),7.234 (d,1H,Ph-H),7.132 (s,1H,Ph-H),2.536 (m,4H,-N==C-CH2-),2.289 (m,4H,-N==C-CH2-),2.143 (s,3H,Ph- CH3),1.595 (m,12H,-CH2-). 2.2. 2-Amino-tert-butyldimethylsilyloxyethane (2) According to the previous work [12],a solution of tertbutyldimethylsilyl chloride (9.06 g,60.0 mmol) in CH2Cl2 (15.0 mL) was added drop-wise under stirring to a solution of ethanolamine (3.63 g,59.0 mmol) and imidazole (7.57 g, 111 mmol) in CH2Cl2 (20 mL). The mixture was allowed to stir at room temperature for 12 h,and then aqueous ammonia (10.0 mL) and CH2Cl2 (50.0 mL) were added. After that,the mixture was washed with brine three times. The organic fraction was dried over MgSO4 and concentrated under reduced pressure. Yield: 70%. 1H NMR (400 MHz,CDCl3): δ 3.573 (t,2H,-CH2-O-), 2.708 (t,2H,NH2-CH2-),1.591 (s,2H,-NH2),0.845 (s,9H,-Si- C(CH3)3),0.012 (s,6H,-Si-CH3); 13C NMR (100 MHz,CDCl3): δ 64.85,44.07,25.93,18.3,-5.31.
2.3. Tolylene-2-tert-butyldimethylsilyloxyethyl carbamide-4- cyclohexanone oxime carbamate (3) A mixture of compounds 1 (2.72 g,6.81 mmol) and 2 (3.01 g, 17.2 mmol) was stirred for 12 h at room temperature in toluene (15.0 mL),and filtered in vacuo. The solid was washed with methanol-toluene (1/3,v/v) three times and a white powder was obtained after vacuum drying. Single crystals grew by allowing water to diffuse into N,N-dimethylformamide solution slowly at room temperature. Yield: 90%. 1H NMR (400 MHz,DMSO-d6): δ 9.431 (s,1H,-NH-) 7.841 (s,1H,-NH-),7.710 (s,1H,Ph-H),7.092 (d,1H,Ph-H),7.023 (d,1H,Ph-H),6.514 (m,1H,-NH-),3.606 (m, 2H,-O-CH2-CH2-NH-),3.165 (m,2H,-O-CH2-CH2-NH-),2.553 (m,2H,-N==C-CH2-),2.287 (m,2H,-N==C-CH2-),2.103 (s,3H,Ph- CH3),1.638 (m,6H,-CH2-),0.871 (s,9H -Si-C(CH3)3),0.049 (s,6H, Si-CH3); 13C NMR (100 MHz,DMSO-d6): δ 160.5,154.5,151.9,135.0,133.1,129.9,129.5,117.1,113.2,63.0,44.7,37.8,31.8,27.7, 25.8,24.0,18.1,14.8,-5.7. 2.4. Tolylene-2,4-hydroxyethyl carbamide (4)
A mixture of compound 1 (2.72 g,6.81 mmol) and ethanolamine (3.24 g,53.1 mmol) was added to toluene (15.0 mL),and the mixture was stirred for 6 h at room temperature,followed by filtration in vacuo. The precipitate was washed with absolute ether three times and a white powder was obtained after vacuum drying.Yield: 65%. 1H NMR (400 MHz,DMSO-d6): δ 8.379 (s,1H,-NH-), 7.701 (s,1H,Ph-H),7.614 (s,1H,-NH-),7.114 (d,1H,Ph-H),6.920 (d,1H,Ph-H),6.615 (m,1H,-NH-),6.035 (m,1H,-NH-),4.690 (m, 2H,-OH),3.423 (m,4H,-CH2-CH2-OH),3.124 (m,4H,-CH2-CH2- OH),2.070 (s,3H,Ph-CH3). 2.5. Tolylene-2,4-n-butyl dicarbamide (5)
n-Butylamine (2.40 g,32.8 mmol) and compound 1 (2.72 g, 6.81 mmol) were added to toluene (15.0 mL),and the mixture was stirred for 12 h at room temperature,followed by filtration in vacuo. The precipitate was washed with absolute ether three times and a white powder was obtained after vacuum drying. Yield: 65%. 1H NMR (400 MHz,DMSO-d6): δ 8.220 (s,1H,-NH-),7.703 (s,1H, Ph-H),7.446 (s,1H,-NH-),7.115 (d,1H,Ph-H),6.917 (d,1H,Ph-H), 6.462 (m,1H,-NH-),5.918 (m,1H,-NH-),3.065 (m,4H,-CH2- CH2-CH2-CH3),2.069 (s,3H,Ph-CH3),1.500-1.200 (m,8H,-CH2- CH2-CH2-CH3),0.911 (t,6H,-CH2-CH2-CH2-CH3). 2.6. Tolylene-2,4-n-butyl dicarbamate (6) n-Butanol (2.43 g,32.8 mmol) and compound 1 (2.72 g, 6.81 mmol) were added to toluene (15.0 mL),and the mixture was stirred for6 h at 50 8C with dibutyltin dilaurate as a catalyst. The mixture was concentrated in vacuo and cyclohexane (15.0 mL) was used to separate precipitate out. The precipitate was washed with absolute ether three times and a white powder was obtained after vacuumdrying. Yield: 50%. 1HNMR (400 MHz,DMSO-d6): δ 9.473 (s1H,-NH-) 8.727 (s,1H,-NH-),7.484 (s,1H,Ph-H),7.126 (t,1H,Ph- H),7.027 (d,1H,Ph-H),4.048(m,4H,-CH2-CH2-CH2-CH3),2.102 (s, 3H,Ph-CH3),1.583 (m,4H,-CH2-CH2-CH2-CH3),1.356 (m,4H, -CH2-CH2-CH2-CH3),0.907 (m,6H,-CH2-CH2-CH2-CH3).
3. Results and discussion
Fig. 2 showed the selective reaction of oxime-blocked 2,4-TDI with amino group. Firstly,2,4-TDI was blocked by reacting with cyclohexanone oxime. Secondly,t-butyldimethylsilyl chloride was used as protecting reagent to react with ethanolamine. The hydroxyl was protected to generate compound 2. Finally,the selective displacement reaction of oxime-blocked 2,4-TDI (1) happened when reacted with compound 2.

|
Download:
|
|
Fig. 2.The selective displacement reaction of oxime-blocked 2,4-TDI: (a) cyclohexanone oxime,toluene,1 h; (b) tert-butyldimethylsilane chloride,imidazole,CH2Cl2,12 h; (c) toluene,12 h. |
|
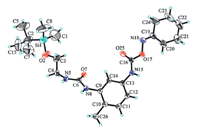
The intervention of siloxane protecting group was very important because it changed the solubility of compound 3. The oxime-blocked 2,4-TDI could be dissolved well in toluene,but compound 3 precipitated from toluene after one of the oxime groups in 1 was substituted by the t-butyldimethylsilyloxyethylamino group. The structure of compound 3 was determined by single-crystal X-ray diffraction as shown in Fig. 3,which provided the direct evidence that only the carbamate ortho to the methyl group reacted.
|
Download:
|
| Fig. 3.Molecular structure of tolylene-2-tert-butyldimethylsilyloxyethyl carbamide-4-cyclohexanone oxime carbamate. | |
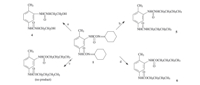
As discussed above,siloxane-protected ethanolamine was the key factor for the high selectivity of the reaction. In order to further confirm the effect of siloxane group,ethanolamine was used to directly react with oxime-blocked 2,4-TDI (Fig. 4a). Obviously, both the groups in oxime-blocked 2,4-TDI reacted,and no selectivity was observed. However,it was very interesting that oxime-blocked isocyanate could only react with amino group when there were both -OH and -NH2 groups in the molecule. In order to further understand that results,n-butylamine and n-butyl alcohol were used as model compounds to react with oximeblocked 2,4-TDI.
To differentiate the reactivity of amines from that of alcohols, many successful intermediates had been developed such as carbonyl azide [13],1,1’-carbonyl diimidazole [14] and cyclic carbonate [15]. We found that 2,4-TDI could also selectively react with n-butyl amine in the presence of n-butyl alcohol after being blocked by cyclohexanone oxime. The reaction rate of oximeblocked 2,4-TDI with n-butyl amine was very fast at room temperature (Fig. 4b),and the reaction of 2,4-TDI with n-butyl alcohol did not occur under the same conditions (Fig. 4c). The latter reaction would happen only at higher temperature (50 ℃) and with a catalyst (dibutyltin dilaurate) (Fig. 4d).
|
Download:
|
| Fig. 4.The selective reaction of oxime-blocked 2,4-TDI with amine and alcohol: (a) ethanolamine,6 h; (b) n-butylamine,12 h; (c) n-butanol,12 h; (d) n-butanol,dibutyltin dilaurate,50 ℃,6 h. | |
The selective derivatization of 2,4-TDI with amine occurs when cyclohexanone oxime is used as blocking agent. The two carbamate groups showed different reactivity toward amine nucleophiles. Moreover,under controlled conditions,oxime-blocked isocyanate can only react with an amino group in the presence of a hydroxy group. This approach can be very useful for the synthesis of novel monomers and polymers with desired structures. Acknowledgment
This work is financially supported by the National Natural Science Foundation of China (Nos. 21176147,21276149 and 21204044) and Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province.
| [1] | (a) I. Oref, Selective chemistry redux, Science 279 (1998) 820-821; (b) J.C. Tully, Mode-selective control of surface reactions, Science 312 (2006) 1004-1005. |
| [2] | D.K. Chattopadhyay, K.V.S.N. Raju, Structural engineering of polyurethane coatings for high performance applications, Prog. Polym. Sci. 32 (2007) 352-418. |
| [3] | L.L. Ferstandig, R.A. Scherrer, Mechanism of isocyanate reactions with ethanol, J. Am. Chem. Soc. 81 (1959) 4838-4842. |
| [4] | P.F. Yang, Y.D. Han, T.D. Li, J.Y. Li, 1H NMR analysis of the tolylene-2,4-diisocyanate-methanol reaction, Chin. Chem. Lett. 21 (2010) 853-855. |
| [5] | (a) D.A. Wicks, Z.W. Wicks Jr., Blocked isocyanate Ⅲ. Part A. Mechanisms and chemistry, Prog. Org. Coat. 36 (1999) 148-172; (b) D.A. Wicks, Z.W. Wicks Jr., Blocked isocyanate Ⅲ. Part B. Uses and applications of blocked isocyanates, Prog. Org. Coat. 41 (2001) 1-83. |
| [6] | (a) C.J. Hawker, R. Lee, J.M.J. Fréchet, One-step synthesis of hyperbanched dendritic polyesters, J. Am. Chem. Soc. 113 (1991) 4583-4588; (b) R. Spindler, J.M.J. Fréchet, Synthesis and characterization of hyperbranched polyurethanes prepared from blocked isocyanate monomers by step-growth polymerization, Macromolecules 26 (1993) 4809-4813; (c) R. Spindler, J.M.J. Fréchet, Two-step approach towards the accelerated synthesis of dendritic macromolecules, J. Chem. Soc. Perkin Trans. 1 (8) (1993) 913-918. |
| [7] | (a) H. Kothandaraman, R. Thangavel, Studies on toluenediisocyanate blocked by benzophenone oximes, J. Polym. Sci. Part A: Polym. Chem. 31 (1993) 2653-2657; (b) H. Kothandaraman, R. Thangavel, Cyclohexanone oxime-blocked polyisocyanates, J. Appl. Polym. Sci. 10 (1993) 1791-1796; (c) H. Kothandaraman, R. Thangavel, Thermal analysis of oxime-blocked toluene diisocyanates, Macromol. Mater. Eng. 207 (1993) 93-99. |
| [8] | (a) K. Schwetlick, R. Noack, F. Stebner, Three fundamental mechanisms of basecatalysed reactions of isocyanate with hydrogen-acidic compounds, J. Chem. Soc. Perkin Trans. 2 (3) (1994) 599-608; (b) K. Schwetlick, R. Noack, Kinetics and catalysis of consecutive isocyanate reactions, formation of carbamates, allophanates and isocyanurates, J. Chem. Soc. Perkin Trans. 2 (2) (1995) 395-402. |
| [9] | (a) R. Gras, E. Wolf, Blockierte isocyanatgruppen und isocyanauratgruppen enthal tende gemische sowie deren herstellung, DE 946085 A1 (1981); (b) R. Gras, J. Obendorf, E. Wolf, Formkoerper-und ueberzugsmassen, DE 3004902 A1 (1981); (c) R. Gras, A. Schott, E. Wolf, Formkoerper-und ueberzugsmassen, DE 3004903 A1 (1981); (d) R. Gras, E. Wolf, Cold-setting solventless thermosetting polyurethane-polyurea molding material, DE 4028288 A (1992); (e) R. Gras, E. Wolf, Blocked (cyclo) aliphatic polyisocyanates and process for their preparation, EP 475003 A1 (1992); (f) R. Gras, E. Wolf, Blocked aliphatic diisocyanates or diisocyanate-adducts, EP 787754 A2 (1997). |
| [10] | (a) C. Zhao, P.F. Yang, S.P. Wang, J.Y. Li, T.D. Li, Synthesis and characterization of asymmetric substituted dicarbamates, Chin. Chem. Lett. 22 (2011) 1167-1170; (b) P.F. Yang, C. Zhao, T.D. Li, Ring-opening and carbamste reaction of uretedione, Polym. Mater. Sci. Eng. 27 (2011) 129-132. |
| [11] | M. Modesti, A. Lorenzetti, An experimental method for evaluating isocyanate conversion and trimer formation in polyisocyanate-polyurethane foams, Eur. Polym. J. 37 (2001) 949-954. |
| [12] | (a) C. Palomo, J.M. Balentova, J. Jimenez, Synthesis of b-lactam scaffolds for ditopic peptidomimetics, Org. Lett. 9 (2007) 101-104; (b) S.B. Larsen, B.B. Andersen, T.N. Johansen, Palladiwm-catalyzed monoamination of dihalogenated benzenes, Tetrahedron 64 (2008) 2938-2950; (c) J.J. Xue, X.M. Yu, Selective 3-and 6-OH modification of (±)-clausenamide, Chin. Chem. Lett. 22 (2011) 761-764. |
| [13] | (a) A. Kumar, S. Ramakrishnan, Hyperbranched polyurethanes with varying spacer segments between the branching pionts, J. Polym. Sci. Part A: Polym. Chem. 34 (1996) 839-848; (b) A. Kumar, E.W. Meijer, Novel hyperbranched polymer based on urea linkages, Chem. Commun. 1 (1998) 1629-1630; (c) A.V. Ambade, A. Kumar, An efficient route for the synthesis of hyperbranched polymers and dendritic building blocks based on urea linkages, J. Polym. Sci. Part A: Polym. Chem. 39 (2001) 1295-1304. |
| [14] | (a) S.P. Rannard, N.J. Davis, The selective reaction of primary amines with carbonyl imidazole containing compounds: selective amide and carbamate synthesis, Org. Lett. 2 (2000) 2117-2120; (b) W.J. Feast, S.P. Rannard, A. Stoddart, Selective convergent synthesis of aliphatic polyurethane dendrimers, Macromolecules 36 (2003) 9704-9706; (c) S.P. Rannard, N.J. Davis, I. Herbert, Synthesis of water soluble hyperbranched polyurethanes using selective activation of AB2 monomers, Macromolecules 37 (2004) 9418-9430. |
| [15] | (a) A.P. Goodwin, S.S. Lam, J.M.J. Fréchet, Rapid efficient synthesis of heterobifunctional biodegradable dendrimers, J. Am. Chem. Soc. 129 (2007) 6994-6995; (b) K. Maruyama, H. Kudo, T. Ikehara, et al., Synthesis and properties of photocross-linkable hyperbranched poly(urethane)s containing both terminal methacryloyl groups and carboxyl groups, Macromolecules 40 (2007) 4895-4900. |




