b College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Ultrafine particles,or NPs,are solid or colloidal particles classified according to their diameter between 1 nm and 100 nm. Since the establishment of colloidal chemistry in 1861,many investigations have been focused on NPs due to their pivotal role in a wide spectrum of advanced applications. The typical sizes of different NPs largely depend on the density and related properties of materials. For instance,metals and metal oxides are easily turned into 1-10 nm particles,with atoms close-packing by mechanical or chemical methods. On the other hand,inorganic materials like SiO2,because of lower density,typically form NPs with a minimum diameter of 15 nm [1]. Due to their thermalsensitivity and viscoelasticity,polymeric NPs (PNPs) below 50 nm are difficult to fabricate. NP research is quickly expanding because of PNPs’ organic compatibility,selective chemical compositions, and suitability in a wide range of application needs. Many efforts have been devoted to the fabrication of PNPs,and several sophisticated methods have been developed through wet-chemical synthesis [2, 3, 4, 5, 6, 7]. Heterogeneous polymerizations,including micro-emulsion polymerization[8],emulsion polymerization under microwave [9],radiation [10] and other specific conditions, and dendrimer based PNPs [11],were popular direct pathways to synthesize PNPs. However,these techniques need expensive apparatuses,complex post-processing to remove additional agents,and can only create PNPs above 50 nm in size and without multifunction.
Functional PNPs with surface reactive groups can be further modified through possible reaction or assembly and have been successfully utilized in the coatings industry [12]. However,PNP preparation ismuchmore complex and difficult to control due to the introduction of active groups. For this reason,there were still few reports on this topic. Recently,Sto¨ver’s group prepared a series of reactive PNPs based on divinyl benzene (DVB) [13, 14, 15, 16]. It was reported that microspheres could be formed by conventional precipitation polymerization of DVB in acetonitrile [13],and poly(divinyl benzene-co-maleic anhydride) (PDVBMAH) in the formsof microspheres,micro-gels andmacro-gels could be obtained through the copolymerization of DVB-55 and maleic anhydride (MAH) at low monomer concentrations in a mixture of n-heptane and methyl ethyl ketone [14, 15]. Nevertheless,the diameters of these obtained polymeric particles were micro-scale (150-600 nm), and the chemical structure and separation process of the PNPs were not discussed in detail. Besides,the solvents used in these studies were highly toxic,noxious and relatively expensive.
According to our previous research [17, 18, 19],polymeric particles with functional groups and size from 100 nm to 800 nm could be directly fabricated through a self-stable precipitation polymerization process,which was free of stabilizer. The reaction system was only constituted of initiator and functional monomers in low toxicity acetate solvent. In this paper,we improve this method and develop a facile method to fabricate reactive copolymer NPs in low-toxicity solvent through self-stable precipitation polymerization of DVB-80 (DVB-80,80% mixture of isomers) and MAH. These NPs were successfully separated from the polymer colloid by precipitation and centrifugation. The SEM results showed that the diameter of these NPs was below 50 nm and FT-IR spectrum indicated that these NPs contain reactive anhydride and vinyl groups. 2. Experimental 2.1. Preparation of PDVBMAH NP
The preparation of PDVBMAH NPs was similar to that of poly(vinyl acetate-alt-maleic anhydride),which has been reported previously [19]. In a typical experiment,the monomers MAH (1.2 mmol) and DVB-80 (2.16 mmol of vinyl groups),the initiator 2,2'-azobis-isobutyronitrile (AIBN) (1 wt% of the monomers),and the harmless solvent n-butyl acetate (n-BA,60 mL) were charged into a 100 mL three-necked flask equipped with a gas inlet tube and a condenser. After all the reagents were dissolved,the solution was purged withN2 for about 15 min,and the flask was then placed in an oil bath under nitrogen atmosphere at 90 ℃ for 2 h. Petroleum ether was used as a precipitant to accelerate the aggregation of the obtained particles. The particle suspension was centrifuged at 4000 r/min for 10 min to separate the resultant PDVBMAH NPs,and the unreacted DVB-80 and MAH remained in the clear centrifugal supernatant. The NPs product was dried under vacuum at room temperature for several days until constant weight was achieved. 2.2. Characterization of PDVBMAH NPs
The SEM and TEM imagines were recorded on a Hitachi S4700 scanning electron microscopy and a Hitachi H800 transmission electron microscopy,respectively. The FT-IR spectra were obtained on a Nicolet Nexus670 spectrometer. The routine iodometric titration was employed to analyze the content of vinyl groups. 3. Results and discussion
The optical image of the resultant dispersion is shown in Fig. 1a. It can be seen that the PDVBMAH NPs formed a homogeneous colloidal suspension with light blue color in n-BA. From the translucent light blue color of the suspension,it can be inferred that the particle size is much smaller. The size of these particles was further characterized by scanning electronic microscopy (SEM) and transmission electronic microscopy (TEM). The SEM image (Fig. 1b1) demonstrated that the copolymer particles were mainly large aggregates with irregular morphology,and the TEM image of these particles (Fig. 1b2) showed ambiguous,isolated outlines,indicating the aggregation of NPs. It was believed that the aggregation of these NPs was most likely due to solvent volatilization and the drying process.

|
Download:
|
| Fig. 1. (a) Photograph of the original colloidal suspension; (b1) SEM image of PDVBMAH NPs; (b2) TEM image of PDVBMAH NPs. | |
In order to further identify the morphology of the copolymer product,two experiments were conducted. In experiment 1,the dried copolymer product was ground into white fine powder (Fig. 2a1),and this powder was redispersed in n-BA in the same concentration as the original colloidal suspension (Fig. 2a2). It can be seen that similar homogeneous dispersion with light blue color was observed,indicating that the PDVBMAH product can be distributed in the form of NPs again to generate a stable translucent colloidal suspension (Fig. 2a2). This result demonstrated that these NPs possessed stable and fixed size and shape, and the precipitation-redispersion process of these NPs is reversible.

|
Download:
|
| Fig. 2. (a1) The dried powder of PDVBMAH NPs; (a2) Photograph of PDVBMAH NPs redispersed in n-BA; (b) SEM image of ionized PDVBMAH NPs with 30 nm diameter. | |
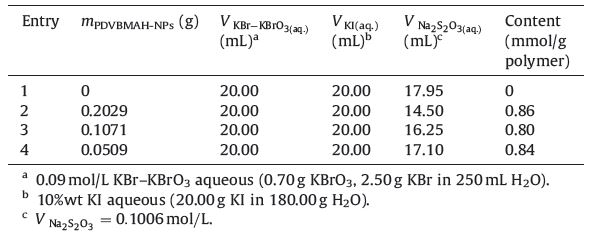
For experiment 2,the NPs were ionized through saponification of the anhydride groups by NaOH,to prevent the aggregation of PDVBMAH NPs during solvent volatilization. The dried copolymer product was added into the appropriate amount of NaOH aqueous solution (pH 14) in a 50 mL beaker at room temperature,and the suspension was exposed to ultrasound for few minutes. The SEM image of ionized PDVBMAH NPs is shown in Fig. 2b,and it can be seen that the NPs dispersed separately without aggregation. The particles isolated each other probably through charge repulsion, which resulted from hydrolysis reaction of anhydride groups with aqueous alkali (Scheme 1). The average diameter of these NPs was about 30 nm and with narrow size distribution.
|
Download:
|
| Scheme 1. Schematic illustration for ionization and dispersion of PDVBMAH NPs. | |
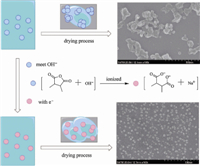
The chemical structure of PDVBMAH NPs was analyzed by FT-IR and the result is shown in Fig. 3. The strong peaks at 1854 cm -1 and 1774 cm -1 were attributed to C=O stretching vibration,and 1073 cm-1 for C-O-C stretching vibration,indicating the presence of anhydride groups. The peak at 3070 cm -1 was ascribed to =C-H stretching vibrations,and characteristic absorption band of pendant vinyl group was at 1646 cm -1,which implied that vinyl groups were present in PDVBMAH NPs (Fig. 3). The peak at 1411 cm- 1 and 1303 cm -1 were due to =C-H scissoring vibration, while other two peaks at 998 cm -1and 919 cm -1 corresponded to =C-H out-of-plane bending vibration. The FT-IR results indicated that these PDVBMAH NPs were functionalized with anhydride groups and pendant vinyl groups attached onto the polymer chain or polymer network,and these anhydride and pendant vinyl groups supplied further reactivity and functionality for PDVBMAH NPs.
|
Download:
|
| Fig. 3. FT-IR spectrum of PDVBMAH NPs. | |
In order to determine the amount of residual vinyl groups in the PDVBMAH NPs,routine iodometric titration was carried out,and the titration result is shown in Table 1. We dispersed different dosages of PDVBMAH NPs into 20 mL distilled water and 5 mL acetone in three parallel entries. In the iodine flask,part of the bromine was consumed by vinyl groups through addition reaction, and the rest was displaced into iodine which could be titrated by Na2S2O3 with starch indicator. The results in Table 1 indicated that the average content of vinyl groups in PDVBMAH NPs was about 0.83 mmol/g. On the assumption that the relative density of this copolymer is 1.00 and the particles are homogeneous spheres with diameter 30 nm,we calculate that there were more than 1000 vinyl groups in each NP.
| Table 1 The content of vinyl groups determined by iodometric titration. |
It is well-known that MAH is a very special monomer which has very low tendency to homopolymerize and very strong liability to undergo alternating copolymerization with styrene derivatives. In our study,DVB-80 with an average functionality of 1.80 was used as the comonomer (1 mol DVB-80 possess 1.80 mol vinyl groups). The vinyl groups of DVB-80 are consumed in three ways: (a) alternative copolymerization with MAH,(b) cross-linking reaction and (c) remaining as pendant vinyl groups. It was supposed that 80% of the monomers were consumed,which meant that 1.73 mmol vinyl groups form DVB-80 (80% of 2.16 mmol) and 0.96 mmol anhydride groups form MAH (80% of 1.2 mmol) participated in the copolymerization process. For DVB,0.96 mmol vinyl groups reacted in an alternative way with MAH. The amount of residual pendant vinyl groups can be calculated according to the results of the iodometric titration. The ‘‘density’’ of the pendant vinyl groups was 0.83 mmol/g and the weight of copolymer product was 0.23 g; in return,there were 0.19 mmol pendant vinyl groups. Consequently,there were 0.58 mmol (1.73-0.96- 0.19 mmol) vinyl groups participating in the cross-linking reaction. In other words,the cross-linking reaction consumed the larger proportion of vinyl groups from DVB-80,so these PDVBMAH NPs should be highly cross-linked.
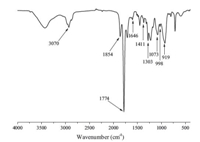
The kinetics of the copolymerization of DVB and MAH was then investigated. The evolution of monomer conversion and particle morphology with reaction time are shown in Figs. 4 and 5, respectively. It is obvious from Fig. 4 that the curve of monomer conversion versus reaction time is similar to that observed in normal radical polymerization. Fig. 5 represents the evolution process of DVB-MAH copolymer product from oligomers to loose colloid particles and finally nanoparticles with increasing monomer conversion. At the beginning of the polymerization (the monomer conversion below 40%),copolymerization of DVB and MAHwas the major reaction,and the resulting oligomers could not lead to the formation of colloid particles due to low molecular weight and insufficient cross-linking. As a result,the reaction solution was transparent without any colors,and no particles appeared in Fig. 5a. With increasing monomer conversion,crosslinking reactions took over the reaction system. As a result,the DVB-MAH oligomers gradually coagulated,crosslinked and formed loose colloid particles as shown in Fig. 5b,and the solution turned slightly blue but still transparent. As the copolymerization and cross-linking reaction generated more and more loose colloid particles and the solvent inside was excluded,the dispersion system gradually changed to be translucent. It can be clearly seen in Fig. 5c that the particle shape was formed (the monomer conversion reached 70%). After the monomer conversion exceeded 70%,the dispersion system showed no further change,but the PDVBMAH NPs with bigger size were obtained in the end of the polymerization (as shown in Fig. 5d).
|
Download:
|
| Fig. 4. Evolution of monomer conversion with reaction time for the copolymerization of DVB and MAH. | |

|
Download:
|
| Fig. 5. Development of PDVBMAH NPs morphologies with reaction time. | |
Based on the above discussion,we proposed a possible mechanism for the formation of PDVBMAH NPs (as shown in Scheme 2). The DVB-80 is a mixture of para-DVB,meta-DVB,paraand meta-ethylvinylbenzene (EVB),and the vinyl groups of DVB, which all have different reactivity. During the self-stable precipitation polymerization process of DVB and MAH,one of DVB’s vinyl groups alternately copolymerized fast with MAH, while the remaining vinyl group with lower reactivity remained as the pendant vinyl groups. These copolymer chains began to precipitate in solution when they propagated long enough. Polymer chain diffusion supplied an opportunity for some of the pendant vinyl groups on one polymer chain to crosslink with those of another polymer chain,leading to the formation of a nucleus. With continual adsorption of newly formed polymer chains,the nuclei excluded inner solvent and grew up to form the highly cross-linked NPs.
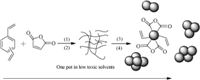
|
Download:
|
| Scheme 2. Schematic illustration for formation mechanism of PDVBMAH-NPs: (1)copolymerizing; (2) cross-linking; (3) precipitating; (4) nucleating. | |
In conclusion,we developed a facile one-pot approach to fabricate reactive polymeric NPs through self-stable precipitation polymerization of DVB and MAH. The size of these obtained PDVBMAH NPs was about 30 nm with narrow distribution,and these NPs were highly cross-linked with reactive surface anhydride and pendant vinyl groups,which provided these particles with the possibility to be further functionalized.
| [1] | R. Birringer, H. Gleiter, H.P. Klein, et al., Nanocrystalline materials an approach to a novel solid structure with gas-like disorder, Phys. Lett. A 102 (1984) 365-369. |
| [2] | (a) S.I. Stupp, V. LeBonoheur, K. Walker, et al., Supramolecular materials: selforganized nanostructures, Science 276 (1997) 384-389; (b) J.P. Rao, K.E. Geckeler, Polymer nanoparticles: preparation techniques and size-control parameters, Prog. Polym. Sci. 36 (2011) 887-913. |
| [3] | J. Pecher, S. Mecking, Nanoparticles of conjugated polymers, Chem. Rev. 110 (2010) 6260-6279. |
| [4] | M.L. Gou, X.L. Zheng, K. Men, et al., Poly(e-caprolactone)/poly(ethylene glycol)/poly(e-caprolactone) nanoparticles: preparation, characterization, and application in doxorubicin delivery, J. Phys. Chem. B 113 (2009) 12928-12933. |
| [5] | F. Bally, D.K. Garg, C.A. Serra, et al., Improved size-tunable preparation of polymeric nanoparticles by microfluidic nanoprecipitation, Polymer 53 (2012) 5045-5051. |
| [6] | A.W. Pan, B.B. Wu, J.M. Wu, Chitosan nanoparticles crosslinked by glycidoxypropyltrimethoxysilane for pH triggered release of protein, Chin. Chem. Lett. 20 (2009) 79-83. |
| [7] | R.G. Chaudhuri, S. Paria, Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications, Chem. Rev. 112 (2011) 2373-2433. |
| [8] | X.J. Xu, L.M. Gan, Recent advances in the synthesis of nanoparticles of polymer latexes with high polymer-to-surfactant ratios by microemulsion polymerization, Curr. Opin. Colloid Interface Sci. 10 (2005) 239-244. |
| [9] | W.M. Zhang, J. Gao, C. Wu, Microwave preparation of narrowly distributed surfactant-free stable polystyrene nanospheres, Macromolecules 30 (1997) 6388-6390. |
| [10] | M. Grasselli, E. Smolko, P. Hargittai, et al., From microspheres to monoliths: synthesis of porous supports with tailored properties by radiation polymerization, Nucl. Instrum. Meth. Phys. Res. B 185 (2001) 254-261. |
| [11] | M. Ogawa, S. Nitahara, H. Aoki, et al., Fluorinated polymer nanoparticles as a novel 19F MRI contrast agent prepared by dendrimer-initiated living radical polymerization, Macromol. Chem. Phys. 211 (2010) 1369-1376. |
| [12] | R.H. Fernando, Nanocomposite and nanostructured coatings: Recent advancements, in: Nanotechnol. Appl. Coat., ACS Symp Ser., American Chemical Society, vol. 1008, Washington, DC, (2009), pp. 2-21. |
| [13] | J.S. Downey, R.S. Frank, W.H. Li, et al., Growth mechanism of poly(divinylbenzene) microspheres in precipitation polymerization, Macromolecules 32 (1999) 2838-2844. |
| [14] | R.S. Frank, J.S. Downey, K. Yu, et al., Poly(divinylbenzene-alt-maleic anhydride) microgels: intermediates to microspheres and macrogels in cross-linking copolymerization, Macromolecules 35 (2002) 2728-2735. |
| [15] | R. Somoghi, D. Donescu, M. Ghiurea, et al., Copolymerization of DVB with MA in non-aqueous dispersion, J. Optoelectron. Adv. Mater. 10 (2008) 1457-1462. |
| [16] | A. Gelir, D.K. Aktas, I. Cianga, et al., Studying the sol-gel transition of styrene-divinyl benzene crosslinking co-polymerization via excimer forming dye molecules, Polymer 47 (2006) 5843-5851. |
| [17] | Q. Yan, Y.W. Bai, Z. Meng, et al., Precipitation polymerization in acetic acid: synthesis of monodisperse cross-linked poly(divinylbenzene) microspheres, J. Phys. Chem. B 112 (2008) 6914-6922. |
| [18] | G.L. Hao, Z.J. Liu, W.T. Yang, et al., The nucleation and particle growth mechanisms of monodisperse microspheres of PSMA in self-stable precipitation polymerization, J. Beijing Univ. Chem. Technol. 37 (2010) 98-102. |
| [19] | C.M. Xing, W.T. Yang, A novel, facile method for the preparation of uniform, reactive maleic anhydride/vinyl acetate copolymer micro-and nanospheres, Macromol. Rapid Commun. 25 (2004) 1568-1574. |