b Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Plant diseases can cause lethal consequences to crops and lead to great losses in agriculture [1]. As a result,agrochemicals play important roles in plant disease control by directly killing the pathogens. However,the increasing applications of agrochemicals have caused serious environmental problems and induced severe drug resistances of pathogens. Therefore,it is of great importance to exploit novel environmentally friendly methods for plant protection. Plant activators that can stimulate the defensive systemof the plant have attractedmuch attention [2, 3, 4]. By activating SAR,a plant can be conferred with long-lasting resistance to a broad-spectrumof pathogens,including viruses,bacteria,fungi,and oomycetes [5, 6]. Several chemicals have been reported to induce SAR efficiently in plants. Among them,S-methyl-benzo[1, 2, 3]thiadiazole-7-carbothioate (BTH),2,6-dichloroisonicotinic acid (INA),and N-cyanomethyl- 2-choloisonicotiamide (NCI) are analog of SA and showed better SAR-inducing activity[7, 8]. As a result,the most successful compound,BTH,has been well exploited for agricultural applications [9, 10, 11, 12, 13]. Similarly,N-(3-chloro-4-methylphenyl)-4-methyl- 1,2,3-thidiazole-5-carboxamide (TDL) and its derivatives are another class of plant activators for the control of rice diseases, which could also induce the expression of SAR marker genes in tobacco [14, 15]. Recently,a novel plant activator,3,4-dichloro-2'- cyano-1,2-thiazole-5-carboxanilide (Isotianil) was developed by Bayer CropScience AG for the control of rice blast. Isotianil showed no antimicrobial activity against various fungi and bacteria,but remarkably displayed high efficacies against these diseases in field tests [16, 17]. Until now,most of the commercialized plant activators bore similar 1,2,3-thiadiazole,or a thiazole moiety in their structures; hence 1,2,3-thiadiazole was the promising pharmacophore for the development of novel plant activators.
Currently,an increasing effort is being put into the study of novel plant activators. However,only a few promising chemicals have been reported to date,and the agricultural applications of plant activators are far from developed [7, 14, 18, 19, 20]. The efficacy of 1,2,3-thidaizoles,as plant activators,has been fully proved, which found that 1,2,3-thidiazole was essential for activity [7, 21]. While in our previous study,7-carboxylate derivatives of BTH have been developed as plant secondary metabolites elicitors and plant activators [22, 23]. The results showed that introduction of fluorine-containing 7-carboxylate esters significantly enhanced the activity of target compounds. Till now,however,much effort has been given to the active study of 1,2,3-thidiazole,and there are only few structure-activity studies on the heterocyclic-fused 1,2,3- thidiazole. Stanetty and colleagues have reported the synthesis of methyl-thieno[2,3-d]-1,2,3-thiadiazole-6-carboxylate and suggested the potential applications as plant activator [24, 25],while no biological activity was investigated. In this study,a series of novel thieno[2,3-d]-1,2,3-thiadiazole-6-carboxylate derivatives were designed and synthesized as novel plant activators. Various ester groups,including fluorine-containing groups,were introduced and their activity as plant activators was evaluated both in vivo and in vitro against several plant diseases. 2. Experimental 2.1. Synthesis of the new compounds
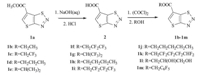
The starting compound 1a was synthesized according to literature report [25]. Compounds 1b-1m were synthesized from 1a via two steps including hydrolization and esterification (Scheme 1). Synthetic procedures of the novel compounds are described in Supporting information,and data of the novel compounds are shown in Tables S1 and S2 (see Supporting information).
|
Download:
|
| Scheme 1. General route for the synthesis of target compounds. | |
In order to screen plant activators efficiently,the assay was conducted in two steps: firstly,all compounds were tested in vivo toward seven pathogens including four types of fungi,two types of bacteria and a type of oomycete. After that,compounds with high activities were selected for the in vitro anti-microbial activity assay. As plant activator,it should protect plants by activating the SAR in vivo and show no direct activity in vitro. Test methods are described in Supporting information. 3. Results and discussion 3.1. Preparation of thieno[2,3-d]-1,2,3-thiadiazole-6-carboxylate derivatives
Compound methyl thieno[2,3-d]-1,2,3-thiadiazole-6-carboxylate (1a) was synthesized according to the literature [25]. Compound 2 was synthesized from 1a via the hydrolysis with good yield,followed by esterification with various alcohols to obtain novel compounds 1b-1m,as shown in Scheme 1. The yields and physical properties of the novel compounds are shown in Table S1. All compounds were characterized by 1H NMR,HRMS,and compounds containing fluorine atoms were also identified by 19F NMR,as shown in Table S2.
Plant activators are a class of chemicals that could activate the defense response of plants being invaded by pathogens and usually have no in vitro fungicidal activity [26]. Therefore,firstly we evaluated the in vivo efficacy of the novel compounds,then in vitro test of the high efficacy compounds was conducted to illustrate the potential as plant activator. Methods of inoculation are described in Table S3 (see Supporting information). The in vivo efficacy of the novel compounds against seven diseases is shown in Table 1. In order to make a better assessment and comparison of the efficacy of the novel compounds,we defined herein the efficacy above 40% as demonstrating active efficacy against the disease,due partly to the fact that positive control,50% procymidone (WP),only showed efficacy of 42% toward Botrytis cinerea. With this standard,we can establish from Table 1 that the positive control BTH was active against four of the seven tested diseases,including Mycosphaerella melonis (88%),Pseudomonas syringae pv. Lachrymans (76%), Phytophthora infestans (65%) and B. cinerea (47%). The free acid of compound 2 only showed efficacy against two of the seven test diseases,including M. melonis (74%) and P. infestans (57%),and that the introduction of various ester groups significantly enhanced the activity,among which,compounds 1d,1k and 1l showed efficacy against three diseases. Compounds 1a,1b, 1f and 1g showed efficacy against four diseases; and especially compound 2,2,2- trifluoroethyl-6-ester (1c) showed efficacy against five of the seven tested diseases,including M. melonis (69%),Corynespora cassiicola (52%),P. syringae pv. Lachrymans (42%),P. infestans (67%),and B. cinerea (41%). In addition,compounds 1a,1b,1c,1f and 1g showed efficacy against various types of pathogens including fungi, oomycetes,and bacteria. Therefore,we concluded that the introduction of the proper ester groups,such as small alkyl groups like methyl (1a) and ethyl (1b),increased the efficacy,while larger alkyl groups,such as propyl (1d) and isopropyl (1e),decreased the activity,as these two compounds only showed activity toward three and two tested diseases,respectively. Furthermore,introduction of fluorine atoms in these alkyl groups could further enhance their activities,as compounds 1c (2,2,2-trifluoroethyl),1f (2,2,3,3,3-pentafluropropyl) and 1g (1,1,1,3,3,3-hexafluoro-2-propyl) all showed better efficacy than 1b (ethyl),1d (propyl) and 1e (isopropyl),respectively.
| Table 1 In vivo induction activity of the target compounds. |
In comparison of the compounds with broad-spectrum of efficacy including 1a,1b,1c,1f and 1g,compound 2,2,2- trifluoroethyl thieno[2,3-d]-1,2,3-thiadiazole -6-carboxylate (1c) showed the broadest spectrum toward the tested diseases,while compound methyl thieno[2,3-d]-1,2,3-thiadiazole-6-carboxylate (1a) had the highest efficacy against the diseases which showed efficacies toward M. melonis (90%),C. cassiicola (77%),P. syringae pv. Lachrymans (41%),and P. infestans (81%). Since only a limited number of compounds were synthesized in this study,the structure-activity relationship could not be fully elucidated,but, in general,we did find a trend that compounds with smaller ester groups showed good in vivo activity and the introduction of fluorine atoms could further enhance the activity,which is in accordance with our previous studies.
Next,in vitro assays were conducted for those compounds with good in vivo efficacy against four micro-organisms,including P. infestans,C. cassiicola,M. melonis and Rhizoctonia solani. The results showed that the novel compounds had almost no fungicidal activity as shown in Table S4 (Supporting information). Surprisingly, the positive control BTH showed efficacies of 20% and 49% against C. cassiicola and R. solani,respectively,while the relevant fungicides,75% chlorothalonil (WP) and 5% validamycin A (WP), showed efficacies of 21% and 50%,respectively,against these two diseases. 4. Conclusion
In this paper,we have developed a series of novel thieno[2,3-d]- 1,2,3-thiadiazole-6-carboxylate derivatives as potential plant activators. The biological activity results illustrated the high efficiency of the novel compounds against a broad-spectrum of pathogens,including bacteria,fungi and oomycetes. Specifically, the 6-methyl ester compound 1a and 6-(2,2,2-trifluoroethyl) ester compound 1c were the most potent candidates among the tested compounds,and showed better efficacy than BTH in our experiments with great potential to be exploited in agriculture as potent plant activators. Acknowledgments
This work is financially supported by the National Basic Research Program of China (973 Program,No. 2010CB126100), the National High Technology Research and Development Program of China (863 Program,No. 2011AA10A207),the China 111 Project (No. B07023),and the Fundamental Research Funds for the Central Universities. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.07.003.
| [1] | L. Bos, Crop losses caused by viruses, Crop Prot. 1 (1982) 263-282. |
| [2] | G. Loake, M. Grant, Salicylic acid in plant defense-the players and protagonists, Curr. Opin. Plant Biol. 10 (2007) 466-472. |
| [3] | J.G. Turner, C. Ellis, A. Devoto, The jasmonate signal pathway, Plant Cell Online 14 (2002) S153-S164. |
| [4] | S.W. Park, E. Kaimoyo, D. Kumar, S. Mosher, D.F. Klessig, Methyl salicylate is a critical mobile signal for plant systemic acquired resistance, Science 318 (2007) 113-116. |
| [5] | J.A. Ryals, U.H. Neuenschwander, M.G. Willits, et al., Systemic acquired resistance, Plant Cell Online 8 (1996) 1809-1819. |
| [6] | I.T. Baldwin, C.A. Preston, The eco-physiological complexity of plant responses to insect herbivores, Planta 208 (1999) 137-145. |
| [7] | W. Kunz, R. Schurter, T. Maetzke, The chemistry of benzothiadiazole plant activators, Pestic. Sci. 50 (1997) 275-282. |
| [8] | L. Sticher, B. MauchMani, J.P. Metraux, Systemic acquired resistance, Annu. Rev. Phytopathol. 35 (1997) 235-270. |
| [9] | K.A. Lawton, L. Friedrich, M. Hunt, et al., Benzothiadiazole induces disease resistance in arabidopsis by activation of the systemic acquired resistance signal transduction pathway, Plant J. 10 (1996) 71-82. |
| [10] | J. GoÉlach, S. Volrath, G. Knauf-Beiter, et al., Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat, Plant Cell Online 8 (1996) 629-643. |
| [11] | N. Benhamou, R.R. Belanger, Benzothiadiazole-mediated induced resistance to fusarium oxysporum f. sp. radicis-lycopersici in tomato, Plant Physiol. 118 (1998) 1203-1212. |
| [12] | N. Benhamou, R.R. Belanger, Induction of systemic resistance to pythium damping-off in cucumber plants by benzothiadiazole: ultrastructure and cytochemistry of the host response, Plant J. 14 (1998) 13-21. |
| [13] | K.A. Ford, J.E. Casida, D. Chandran, et al., Neonicotinoid insecticides induce salicylate-associated plant defense responses, Proc. Natl. Acad. Sci. U.S.A. 107 (2010) 17527-17532. |
| [14] | M. Yasuda, H. Nakashita, S. Yoshida, Tiadinil, a novel class of activator of systemic acquired resistance, induces defense gene expression and disease resistance in tobacco, J. Pestic. Sci. 29 (2004) 46-49. |
| [15] | Z.J. Fan, Z.G. Shi, H.K. Zhang, et al., Synthesis and biological activity evaluation of 1,2,3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance, J. Agric. Food Chem. 57 (2009) 4279-4286. |
| [16] | M. Ogawa, A. Kadowaki, T. Yamada, O. Kadooka, Applied development of a novel fungicide Isotianil, Sumitomo Kagaku (Osaka, Japan) 1 (2011) 4-17. |
| [17] | C.M.J. Pieterse, A. Leon-Reyes, S. Van der Ent, S.C.M. Van Wees, Networking by small-molecule hormones in plant immunity, Nat. Chem. Biol. 5 (2009) 308-316. |
| [18] | M. Iwata, Probenazole-a plant defence activator, Pestic. Outlook 12 (2001) 28-31. |
| [19] | M. Nishioka, H. Nakashita, H. Suzuki, et al., Induction of resistance against rice blast disease by a novel class of plant activator, pyrazolecarboxylic acid derivatives, J. Pestic. Sci. 28 (2003) 416-421. |
| [20] | F. Gozzo, Systemic acquired resistance in crop protection: from nature to a chemical approach, J. Agric. Food Chem. 51 (2003) 4487-4503. |
| [21] | M. Yasuda, M. Kusajima, M. Nakajima, et al., Thiadiazole carboxylic acid moiety of tiadinil, SV-03, induces systemic acquired resistance in tobacco without salicylic acid accumulation, J. Pestic. Sci. 31 (2006) 329-334. |
| [22] | Y.F. Xu, Z.J. Zhao, X.H. Qian, et al., Novel, unnatural benzo-1,2,3-thiadiazole-7-carboxylate elicitors of taxoid biosynthesis, J. Agric. Food Chem. 54 (2006) 8793-8798. |
| [23] | Q.S. Du, W.P. Zhu, Z.J. Zhao, X.H. Qian, Y.F. Xu, Novel benzo-1,2,3-thiadiazole-7-carboxylate derivatives as plant activators and the development of their agricultural applications, J. Agric. Food Chem. 60 (2012) 346-353. |
| [24] | P. Stanetty, M. Kremslehner, H. Vollenkle, A new type of plant activator: synthesis of thieno[2,3-d][1,2,3]thiadiazole-6-carboxylic acid derivatives via Hurd-Mori cyclization, J. Chem. Soc. Perkin Trans. 1 (1998) 853-856. |
| [25] | P. Stanetty, E. Gorner, M.D. Mihovilovic, An improved synthetic approach to thieno[2,3-d]-1,2,3-thiadiazole-carboxylates via diazotization of aminothiophene derivatives, J. Heterocycl. Chem. 36 (1999) 761-765. |
| [26] | H. Kessmann, T. Staub, J. Ligon, M. Oostendorp, J. Ryals, Activation of systemic acquired disease resistance in plants, Eur. J. Plant Pathol. 100 (1994) 359-369. |





