1. Introduction
Dendrimers have been extensively explored in the past two decades because of their controllable nanosize and structure,multivalency,physicochemical properties,and biological activities [1]. Particularly,peptide dendrimers,showing good biocompatibility and biodegradability,as well as protein-like architectures,have been widely studied in supramolecular self-assembly and biomedical applications,such as drug and gene carriers [2, 3, 4]. General approaches for the synthesis of dendrimers were divergent,convergent,and divergent-convergent routes. However,the structural defects increase with the increasing of the generations of dendrimers in the divergent approach,while the intact conjugation or incomplete coupling of the dendritic core and dendritic branches usually exist in both convergent and divergent-convergent approaches. Highly efficient copper-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry [5] could be used to overcome the problems in the synthesis of dendrimers.
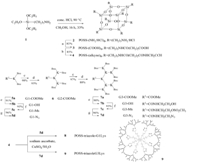
In the synthesis of peptide dendrimers with organic cores,the increase of branches would add to the difficulty as more monomers were conjugated to receive the dendrimers with perfect architectures. Interestingly,polyhedral oligomeric silsesquioxane (POSS),an inorganic nano-cubic molecule with eight functionalities extending outward in three-dimensions has attracted more and more attention as the core for dendrimer synthesis,as more peripheral functional groups in the dendrimers were achieved without high generations. This form of inorganic- organic composite dendrimer has shown great potential in biomedical applications,such as drug and gene delivery [6, 7]. However,the synthesis of POSS-(NH2·HCl)8 core was timeconsuming where 5-10 days,or even several weeks,were required by traditional preparation [8]. Moreover,the divergent approach to synthesize those POSS based peptide dendrimers, which was reported in previous studies,has shown molecular defects with increasing generations. Herein,we adopted a divergent-convergent approach using copper-catalyzed azide- alkyne click chemistry (Fig. 1),which was widely used because of its high selectivity and reactivity. Firstly,dendritic peptides were synthesized by the divergent approach. The peptide dendrimers were then prepared by the click chemistry between alkynefunctionalized POSS and azido-functionalized dendritic peptides. The preparation time of POSS-(NH2·HCl)8 was reduced remarkably by increasing the reaction temperature.
|
Download:
|
| Fig. 1. Schematic illustration of POSS based poly(L-lysine) dendrimer.Reaction conditions: (a) Et3N,CH3OH; (b) 2-propynylamine,HOBT,HBTU,and DIPEA; (c) TFA; (d) BocLys(Boc)-OH,HOBT,HBTU,and DIPEA; (e) ethanolamine,55℃; (f) MsCl,TEA; and (g) NaN3,100℃. | |
Reagents (3-aminopropyl)-triethoxysilane (1) and 2-propynylamine (98%) were purchased from Sigma-Aldrich (Shanghai, China). Succinic anhydride,ethanolamine,triethylamine (TEA), N,N-diisopropylethylamine (DIPEA),trifluoroacetic acid (TFA), sodium azide,CuSO4·5H2O,and sodium ascorbate were obtained from Asta Tech Pharmaceutical (Chengdu,China). 2-(1H-Benzotriazole-1-yl)-1,1,3,3-teramethyluronium hexafluorophosphate (HBTU) andN-hydroxybenzotriazole (HOBT) were obtained from GL Biochem (Shanghai,China). All the organic solvents used in this study were purified using the standard method and distilled before use,if there were no specific directions.
Synthesis of 2: To a solution of methanol (350 mL,chemical pure) and concentrated HCl (30 mL),(3-aminopropyl)-triethoxysilane (15 mL,60 mmol) was added dropwise at 60℃,and then the mixture temperature was increased to 90℃ for 16 h. After cooling down to r.t.,THF (300 mL,chemical pure) was added to precipitate and the white precipitation was concentrated by centrifugation, followed by washing with THF. The organic solvents were removed and 2 was obtained as white powder,yield 33%.
Synthesis of 3 can be found in our previous report [2].
Synthesis of 4:To 2 gof 3,0.92 g HOBT,2.68 g HBTU in freshly distilled DMF was added 0.5 mL 2-propynylamine and 3 mL DIPEA in DMF. The mixture was stirred for 24 h under N2 atmosphere. The concentrated solution was washed with 0.5 mol/L NaHSO4,10% NaHCO3,and saline. The organic phase was then dried with anhydrous MgSO4. Acetonitrile was added to precipitate 4 as a white solid. Compound 4 was then obtained by vacuum drying with a yield of 85%.
Synthesis of 5a,6,and 7a can be found in our previous research [9]. Synthesis of 5x(x=b,c,or d),was similar to that of corresponding 7x. Differently,the purification of 5x was carried out by silica column chromatography (eluent: CHCl3/EtOAc/EtOH, 6:2:1).
Synthesis of 7b: To 2.50 g of 7a in methanol was added 5 mL ethanolamine. The mixture was stirred for 48 h at 55℃ under N2 atmosphere. The solvent was concentrated and precipitated with citric acid aqueous solution. The filtrate was washed with distilled water and dried under vacuum to obtain 7b,yield 90%.
Synthesis of 7c: To a solution of 7b(1.76 g) in CH2Cl2(100 mL) was added MsCl (0.4 mL) and TEA (1 mL) in an ice bath. The mixture was stirred for 24 h at r.t. The organic phase was washed with NaHCO3(20 mL×3) and brine (20 mL×3),and dried with anhydrous MgSO4. 7c was then obtained by vacuum drying with a high yield of 93%.
Synthesis of 7d: To 1.34 g of 7c in DMF (20 mL) was added sodium azide (NaN3,0.24 g). The solution was stirred for 24 h at 100℃. Concentrated solution was re-dissolved in CH2Cl2,washed with brine,and dried with anhydrous MgSO4. 7d was obtained by vacuum drying with a yield of 96%.
Synthesis of 9: A mixture of the 7d(300 mg) and 4(42 mg) in DMF-H2O (4:1,10 mL) in the presence of 5 mol % CuSO4·5H2O with 10 mol % sodium ascorbate was stirred at r.t. for 2 days at 50℃. Solvents were removed by vacuum distillation and the oil crude product was dissolved in CDCl3. The solution was washed by NaHSO4 (30 mL×3),EDTA solution (30 mL×3),and brine (30 mL×3) and dried with anhydrous MgSO4. The CDCl3 solution was concentrated and added to acetonitrile (100 mL). The resulting precipitate was filtered and dried in vacuum to constant weight. Copper was removed by dialysis against dilute EDTA solution and deionized water,yield 75%.8 was handled similarly with a yield of 88%.
Deprotection of 8 and 9: Taking 9 as an example,to a solution of 9 (100 mg) in CH2Cl2(2 mL) was added TFA (200mL) in an ice bath. The reaction was stirred for 6 h at r.t. Ethyl ether was added to precipitate and the precipitate was dried under vacuum with a high yield of 95%. Deprotection of 8 was similar.
The product from deprotection of 8 and 9 was dissolved in water and the pH was tuned to be basic by NaHCO3 solution. The solution was then dialysis against deionized water to remove salts and the result solution was used for dynamic light scattering (DLS, Malvern Zetasizer Nano ZS) test directly.
1H NMR spectra were recorded on a Bruker Avance II NMR spectrometer at 400 MHz. Fourier transform infrared (FTIR) spectra were recorded on a Perkin-Elmer spectrophotometer. Gel permeation chromatography (GPC) measurements were carried out using a Waters instrument that was equipped with a model 515 pump,where THF was used as fluent with a speed of 0.35 mL/min at 40℃.
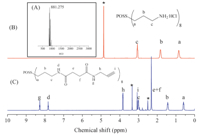
3. Results and discussionIn the synthesis of the POSS core,we determined that a more rapid synthesis of POSS-(NH2·HCl)8 (2) could be achieved by increasing the reaction temperature from room temperature to 90℃. The reaction time was reduced from 5-10 days to only 16 h while the yield was not changed (33% vs.traditional 30%). The successful synthesis of 2 was demonstrated by MS as shown in Fig. 2A. The molecular peak was observed at m/z 881 ([M+H]+). The obtained POSS was also confirmed by 1H NMR and FTIR. As shown in Fig. 2B,the signals at δ 0.6,1.9,and 3.2 were assigned to the protons adjacent to silicon atoms extending outward. The FTIR spectrum (Fig. S1 in Supporting information) showed a characteristic absorbance peak at 1123 cm-1 which corresponded to the stretching vibration of Si-O-Si in the POSS matrix.
|
Download:
|
| Fig. 2. (A) MS spectrum of 2 and 1H NMR spectra of (B) 2 and (C) 4 in D2O and DMSO-d6,respectively. | |
The POSS was then alkyne-functionalized by two steps. Succinic anhydride was first used to react with the amine group on 2 to receive POSS-(COOH)8(3),it was then reacted with 2-propynyl amine to synthesize POSS-(alkyne)8(4). The 1H NMR spectrum of 4 is shown in Fig. 2C. The peaks at δ 3.0 and 0.6 were ascribed to the alkyne protons and α-protons adjacent to POSS,respectively. The area ratio of the two peaks was 1:2,indicating that the POSS was completely alkynated. Notably,we had also tried another method to synthesize alkynated POSS by reacting 2 with propargyl bromide. However,the result was unsuccessful due to the poor solubility of 2 in most organic solvents,and the basicity of the reaction broke the Si-O-Si bond in the POSS matrix.
Dendritic poly(L-lysine) with generations from 1 to 3 was synthesized by the divergent approach as previously reported [9]. Azido-functionalized poly(L-lysine) dendrimers with generations 1 and 3 (G1-N3 and G3-N3) were synthesized by aminolysis, mesylation and finally azidotion of the corresponded dendrimers (5a and 7a),which had the core carboxylic acid protected by methanol ester and the peripheral amine group protected bytertbutoxylcarbonyl (Boc). Ethanolamine was used to aminolysis in 55℃ [10]. The hydroxy group was converted to the ester group (- Ms),which could be azided easily. The overall yield was about 80%. Taking G3-N3 for example,the successful azidotion was directly confirmed by FTIR spectrum (Fig. S2B in Supporting information). There was a characteristic peak at 2100 cm-1 which corresponded to the typical stretching vibration absorbance of N3.
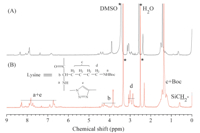
The POSS based generation 1 (8) and 3 (9) poly(L-lysine) dendrimers were then synthesized via CuAAC click chemistry where DMF-water solution (1:1,v/v) with 5% mol CuSO4·5H2O and 10% mol sodium ascorbate used as catalyst. The reaction mixture was precipitated with acetonitrile and dialyzed against dilute EDTA solution and deionized water to remove copper. The obtained dendrimer showed no peaks at 2100 cm-1 in FTIR spectrum (Fig. S2C in Supporting information),suggesting that alkyne and azide groups reacted completely and clearly removed. The 1H NMR spectra of 8 and 9 are shown in Fig. 3,and characteristic peaks of both POSS and amide bond in dendritic lysine were observed. The structures of the dendrimers were further confirmed by GPC (Fig. S3 in Supporting information). The dendrimers of G1 and G3 both showed very narrow distribution with low polydispersity of 1.03 and 1.06,respectively.
|
Download:
|
| Fig. 3. 1H NMR spectra of POSS based poly(L-lysine) dendrimers (A) 8 and (B) 9. | |
The deprotection of peripheral Boc groups on 8 or 9 was carried out in CH2Cl2/TFA solution. The resulting peptide dendrimers,POSS-triazole-G1lys(NH2)16 and POSS-triazole-G3lys(NH2)64,were water soluble and their hydrodynamic diameters were investigated by dynamic light scattering (DLS,Fig. S4 in Supporting information). The mean diameters were less than 10 nm and the dendrimers were monodisperse. The G3 showed a larger size than G1. The POSS core itself was about 1 nm and the size of dendrimers with POSS cores increased after the peptide dendrimers were immobilized on POSS by click reaction. The higher generation dendrimer was conjugated,the thicker shell was received on POSS core.
4. ConclusionPoly(L-lysine) dendrimers with POSS cores were synthesizedvia Cu-catalyzed azide-alkyne cycloaddition click chemistry. It is a facile approach to prepare peptide dendrimers with perfect architectures.
AcknowledgmentThis research work was supported by the National Basic Research Program of China (National 973 program,No. 2011CB606206), National Science Foundation of China (NSFC,Nos. 31170921, 51133004),the National Science Foundation for Excellent Young Scholars (No. 51222304),Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1163).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.06.015.
| [1] | L. Crespo, G. Sanclimens, M. Pons, et al., Peptide and amide bond-containing dendrimers, Chem. Rev. 105 (2005) 1663-1682. |
| [2] | H. Yuan, K. Luo, Y. Lai, et al., A novel poly (L-glutamic acid) dendrimer based drug delivery system with both pH-sensitive and targeting functions, Mol. Pharm. 7 (2010) 953-962. |
| [3] | Z. Gu, K. Luo, W. She, et al., New-generation biomedical materials: peptide dendrimers and their application in biomedicine, Sci. China Chem. 53 (2010) 458-478. |
| [4] | X. Xu, H. Yuan, J. Chang, et al., Cooperative hierarchical self-assembly of peptide dendrimers and linear polypeptides into nanoarchitectures mimicking viral capsids, Angew. Chem. 124 (2012) 3184-3187. |
| [5] | P. Wu, A.K. Feldman, A.K. Nugent, et al., Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes, Angew. Chem. Int. Ed. 43 (2004) 3928-3932. |
| [6] | Y. Pu, S. Chang, H. Yuan, et al., The anti-tumor efficiency of poly (L-glutamic acid) dendrimers with polyhedral oligomeric silsesquioxane cores, Biomaterials 34 (2013) 3658-3666. |
| [7] | T.L. Kaneshiro, X. Wang, Z.R. Lu, Synthesis, characterization, and gene delivery of poly-L-lysine octa (3-aminopropyl) silsesquioxane dendrimers: nanoglobular drug carriers with precisely defined molecular architectures, Mol. Pharm. 4 (2007) 759-768. |
| [8] | J.J. Schwab, J.D. Lichtenhan, Polyhedral oligomeric silsesquioxane (POSS)-based polymers, Appl. Organomet. Chem. 12 (1998) 707-713. |
| [9] | R. Zhu, W. Jiang, Y. Pu, et al., Functionalization of magnetic nanoparticles with peptide dendrimers, J. Mater. Chem. 21 (2011) 5464-5474. |
| [10] | Y. Li, Q. Li, F. Li, et al., Amphiphilic poly(L-lactide)-b-dendritic poly(L-lysine)s synthesized with a metal-free catalyst and new dendron initiators: chemical preparation and characterization, Biomacromolecules 7 (2006) 224-231. |




