1. Introduction
Confinement effects of porous media have significant influence on the phase behaviors of liquids [1]. In the past decades, thermodynamic properties of the confined pure liquids have been extensively investigated [2, 3]. Gubbins and coworkers discovered that the transition temperature of pure liquid under confinement is driven by the ratio of wall/liquid and liquid/liquid interactions [4, 5]. At present,few studies have been reported on phase behavior of confined binary mixtures. It has been reported that within silica gel (15 nm) a CaCl2-H2O mixture shows a same eutectic system with the bulk [6]. A similar phenomenon is observed for C6H5Br-CCl4 mixtures adsorbed in CPGs with a pore diameter of 7.5 nm [7]. In our systematic work,pore size shows significant effect on phase behaviors of n-C10H22-C12H26,n-C12H26-C14H30 and n-C11H24-C12H26 binary mixtures adsorbed in SBA-15 [8, 9, 10].
In alkane family,undecane (C11) and tetradecane (C14) have medium chain lengths with different molecular symmetry. In the low temperature stable form,undecane crystal has an orthorhombic unit cell belonging to space groupPbcmwith dimensions a= 4.97Å ,b= 7.48 Å ,and c= 31.76 Å . Tetradecane crystal has a triclinic unit cell in space group P¯ 1(Z= 1) with dimensions a= 4.29 Å ,b= 4.82 Å ,and c= 19.84 Å [11]. According to the literature,undecane possesses a stable rotator phase while tetradecane does not [12]. As a porous medium,SBA-15 has cylindrical channels with hexagonal arrangements. The pore size of SBA-15 can be adjusted from about 3 nm up to 20 nm and thus is a good porous matrix for the study [13, 14].
In this paper,we propose an investigation of the phase behavior of C11-C14 mixtures in bulk and confined in SBA-15 (3.8,7.8,and 17.2 nm) using DSC. The bulk mixtures show specific behavior relating to rotator phase. Under confinement,phase diagrams of the mixtures vary with pore size as,temperature,and mole fractions.
2. ExperimentalSBA-15 with pore sizes of 3.8,7.8 and 17.2 nm was prepared according to the literature methods,in which triblock copolymer Pluronic P123 was a template for pores and TIPB as a micelle expander for large pore SBA-15 (17.2 nm) [9, 10, 15]. SBA-15 powders with a mass of about 10 mg were put in a glass tube and degassed at 423 K under a vacuum of 10-1 Torr for 2 h. Then a certain amount of C11,C14 or the mixture was transferred to the tube under a pure nitrogen atmosphere through a glass capillary. The volume of alkanes was taken as 95-100% of the pore volume of SBA-15 in order to avoid the interference of the excess liquids. Finally,the glass tube was sealed and equilibrated at room temperature for 3 h.
The sample of SBA-15 adsorbed with alkanes was put in a DSC aluminum pan and sealed immediately. Then it was analyzed on TA DSC Q10 under a high purity nitrogen atmosphere with a scanning rate of 5 K min-1. The temperature scale of the instrument was calibrated with high purity indium and pure water. The mass of the samples for DSC analysis is about 3-4 mg. In most cases the transition temperature variation was within 0.5 K.
3. Results and discussionThe DSC curves of the C11-C14 mixtures in the bulk and confined in SBA-15 are shown in Fig. 1. The bulk C11-C14 mixtures exhibit multiple peaks in mole fraction xC14 =0.1-0.9 (Fig. 1a). Two quasi-invariant temperatures appear at 230.6 K and 244.4 K. Confined inside SBA-15 (3.8 nm and 7.8 nm),C11-C14 mixtures display only one endothermic peak at each composition. In the larger pores of SBA-15 (17.2 nm),one additional thermal anomaly appears in the range of xC14 =0-0:6 besides the melting peak. It is clear that the phase behavior of the confined mixtures becomes more complicated as the pore size increases.
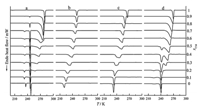
|
Download:
|
| Fig. 1. DSC curves of C11H24-C14H30 mixtures (xC14) in bulk (a) and confined in SBA-15 of pore size: 3.8 nm (b),7.8 nm (c) and 17.2 nm (d). | |
According to the DSC scans,the solid-liquid phase diagram of the bulk C11-C14 system was established in reference to the shape factor method [16],shown in Fig. 2a. For the confined mixtures, phase diagrams are depicted on connecting onset points of the endothermic peaks in each system (Fig. 2b-d). The dotted lines in Fig. 2d are extrapolated from the possible trends of the series phase transitions. Phases in the diagram of the bulk system are assigned on the basis of the general rules of organic crystals relating to molecular packing density and symmetry,as well as its specific properties such as rotator phase. The composition and solid structures of the pore alkanes are estimated in reference to the bulk system and the effect of pore confinement.
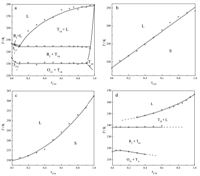
|
Download:
|
| Fig. 2. Experimental solid-liquid phase diagram of bulk C11-C14 system (a),and confined in SBA-15 (3.8 nm) (b),SBA-15 (7.8 nm) (c) and SBA-15 (17.2 nm) (d). T14 represents triclinic crystal of pure C14 or its solid solution,RIthe rotator phase,O13 orthorhombic crystal of pure C13 or its solid solution. The dotted lines indicate the possible trends. The solid phases are assigned on basis of the knowledge of bulk binary system of normal alkanes. | |
In Fig. 2a,the bulk system displays rich phase regions of typical normal alkane mixtures because of the special rotator phase RI. Among them,two-phase regions include (RI + L),(T14+ L), (RI +T14),and (O11+T14) and one-phase regions are RI,O11,and T14. Here,RI,O11,and T14 represent rotator phase,orthorhombic and triclinic crystals of pure C11,C14 and also their solid solutions by C11 and C14. This is a partially miscible binary system,which contains two quasi-invariants,a eutectic point at around 244.4 K and a peritectic point at 230.6 K. As a feature of the chain molecules,the rotator phase region RI covers a wide range of compositions of xC14 =0-0.9. Although C14 has no stable rotator phase,the RI solid solution in this system could be stabilized by mixing,which is observed in other normal alkane mixtures [17]. The phase diagram of the bulk system is a bit simpler compared with those with two carbon difference in the chains.
Confined in pores of SBA-15 (3.8 nm),C11-C14 mixtures display a melting boundary of straight line (Fig. 2b). It seems to be the melting of an ideal solid solution of a complete miscible binary system,in which the melting point is simply proportional to the mole fraction. That is,two components with complete miscibility might be responsible for this melting behavior,according to the knowledge of the bulk systems. In the bulk,only a few binary mixtures possess such a behavior in all the compositions (e.g.the melting of rotator phase RII in C23-C24 system) [18]. The dramatic change in the phase behavior of the pore alkanes indicates a significant influence on the alkane structures from the nanoconfinement.
Inside SBA-15 (7.8 nm),C11-C14 mixtures exhibit a curved line by the melting points (Fig. 2c). Although a bit different in the boundary shape,this system should have a similar behavior to that in SBA-15 (3.8 nm). Nonetheless,the melting of the mixtures in this system could not come from two or more kinds of crystals with different compositions. The possible way for the melting process might result from a eutectoid or solid solution,where the compositions allow such melting behavior.
In the larger pores of SBA-15 (17.2 nm),three curves from melting points and solid-solid transition temperatures could divide the solid phase into three regions. Guided with the dotted lines,the three sections roughly correspond to some regions in the bulk,e.g. (T14+ L),(RI +T14),and (O11+T14),which might be assigned to the confined phases as shown in Fig. 2d. Following this, the pore solid might experience transitions O11→RI→L and T14→L in some compositions,which is consistent with the thermal anomalies in DSC scans. Although the other details of the bulk cannot be detected in DSC scans,the confined mixtures in this larger pore show more phase behaviors than those in the smaller pores.
As the components of the mixtures,C11and C14 also show their melting behaviors as a function of pore size. Their melting points are shifted down to lower temperatures as indicated by the DSC scans. The shift can be qualitatively related to the inverse pore diameter,which could be understood on the basis of the work of Gubbins and coworkers (Eq. (1)) [1] and a modified G-T equation [19] (Eq. (2)) as follows:
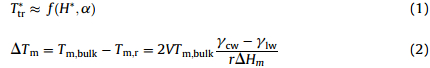
In order to better interpret the phase behavior of the confined mixtures,an understanding of the state of the pore solids would be helpful. In the bulk solid,the pure or mixed alkanes possess layered structures with molecular chains parallel to each other within a layer. Inside pores of controlled porous glass (CPG),however,Knorr and coworkers have found the quenching of lamellar ordering of pore C19 in the solid state [20]. The microspheres of C18,C20 or their mixtures show also quenched or weakened layered ordering [21].Therefore,it is reasonable to assume the pore alkanes exist actually in 2D close-packed arrangements of molecules confined in SBA-15, which are responsible for the dramatic change in the phase behavior. In such a system,the phase behavior should be much different from that of the bulk otherwise 3D structure would be needed as in the cases of the normal binary mixtures. Indeed,the distinct phase diagrams of the confined C11-C14 system compared to that of the bulk show clearly the dependence on the physical size as well as the temperature and mole fraction.
4. ConclusionPore size shows great influence on the phase behavior of C11- C14 binary mixtures confined in SBA-15. Inside SBA-15 (3.8 nm and 7.8 nm),the mixtures show simple single lines of the melting boundaries. In SBA-15 (17.2 nm),the mixtures exhibit more,but still far fewer than the bulk,phase behaviors The 2D close-packed arrangements of molecules are assumed to be responsible for the phase behaviors of the solid pore alkanes. In future experiments, more techniques such as XRD and NMR will be used to characterizethe existing states of pore alkanes to gain a deeper understanding of these new physical phenomena.
AcknowledgmentWe thank the financial support from National Natural Science Foundation of China (No. 21273138) and Natural Science Foundation of Shandong Province,China (No. ZR2010BM035).
| [1] | C. Alba-Simionesco, B. Coasne, G. Dosseh, et al., Effects of confinement on freezing and melting, J. Phys. Condens. Matter 18 (2006) R15-R68. |
| [2] | H.K. Christenson, Confinement effects on freezing and melting, J. Phys. Condens. Matter 13 (2001) R95-R133. |
| [3] | M. Alcoutlabi, G.B. McKenna, Effects of confinement on material behaviour at the nanometre size scale, J. Phys. Condens. Matter 17 (2005) R461-R524. |
| [4] | B. Coasne, J. Czwartos, M. Sliwinska-Bartkowiak, K.E. Gubbins, Effect of pressure on the freezing of pure fluids and mixtures confined in nanopores, J. Phys. Chem. B 113 (2009) 13874-13881. |
| [5] | B. Coasne, J. Czwartos, M. Sliwinska-Bartkowiak, K.E. Gubbins, Freezing of mixtures confined in silica nanopores: experiment and molecular simulation, J. Chem. Phys. 133 (2010) 084701-84709. |
| [6] | Y.L. Aristov, G.D. Marco, M.M. Tokarev, et al., Selective water sorbents for multiple applications, CaCl2 solution confined in micro-and mesoporous silica gels: pore size effect on the "solidification-melting" diagram, Catal. Lett. 61 (1997) 147-154. |
| [7] | J. Czwartos, M. Sliwinska-Bartkowiak, B. Coasne, K.E. Gubbins, Melting of mixtures in silica nanopores, Pure Appl. Chem. 81 (2009) 1953-1959. |
| [8] | H.R. Pei, X. Yan, X.Z. Lan, Unusual phase behavior of decane-dodecane mixtures confined in SBA-15: size effect on binary phase diagram, Chin. Chem. Lett. 23 (2012) 1173-1176. |
| [9] | X.Z. Lan, H.R. Pei, X. Yan, W.B. Liu, Phase behavior of dodecane-tetradecane binary system confined in SBA-15, J. Therm. Anal. Calorim. 110 (2012) 1437-1442. |
| [10] | X. Yan, H.R. Pei, T.B.Wang, W.B. Liu, X.Z. Lan, Phase behavior of undecane-dodecane mixtures confined in SBA-15, J. Chem. 2013 (2013), Article ID 476236, 7 pp. |
| [11] | S.R. Craig, G.P. Hastie, K.J. Roberts, J.N. Sherwood, Investigation into the structures of some normal alkanes within the homologous series C13H28 to C60H122 using high-resolution synchrotron X-ray powder diffraction, J. Mater. Chem. 4 (1994) 977-981. |
| [12] | P. Huber, V. Soprunyuk, K. Knorr, Structural transformations of even-numbered nalkanes confined in mesopores, Phys. Rev. E 74 (2006) 031610-31615. |
| [13] | Y.Y. Pu, Y. Li, W. Zhuang, et al., Preparation and characterizations of helical mesoporous silica nanorods using CTAB and alcohols, Chin. Chem. Lett. 23 (2012) 1201-1204. |
| [14] | Z.L. Yang, J.L. Li, C.L. Zhang, Y.F. Lu, Z.Z. Yang, Two-dimensional mesoporous materials: from fragile coatings to flexible membranes, Chin. Chem. Lett. 24 (2013) 89-92. |
| [15] | L. Cao, T. Man, M. Kruk, Synthesis of ultra-large-pore SBA-15 silica with twodimensional hexagonal structure using triisopropylbenzene as micelle expander, Chem. Mater. 21 (2009) 1144-1153. |
| [16] | T. Calvet, E. Tauler, M.A. Cuevas-Diart, et al., Miltenburg, application of the "shape-factors method" to purity analysis of compounds by thermal methods, Thermochim. Acta 204 (1992) 271-280. |
| [17] | D. Mondieig, F. Rajabalee, V. Metivaud, N-Alkane binary molecular alloys, Chem. Mater. 16 (2004) 786-798. |
| [18] | A. Sabour, J.B. Bourdet, M. Bouroukba, M. Dirand, Modifications to the binary phase diagram of the alkane mixtures n-C23-n-C24, Thermochim. Acta 249 (1995) 269-283. |
| [19] | G. Dosseh, Y. Xia, C. Alba-Simionesco, Cyclohexane and benzene confined in MCM-41 and SBA-15 confinement effects on freezing and melting, J. Phys. Chem. B 107 (2003) 6445-6453. |
| [20] | P. Huber, D. Wallacher, J. Albers, K. Knorr, Quenching of lamellar ordering in an nalkane embedded in nanopores, Europhys. Lett. 65 (2004) 351-357. |
| [21] | D. Fu, Y. Liu, Y. Su, G. Liu, D. Wang, Crystallization behavior of binary even-even nalkane mixtures in microcapsules: effect of composition and confined geometry on solid-solid phase separation, J. Phys. Chem. B 115 (2011) 4632-4638. |




