The upsurge in green chemistry has inspired the fraternity of researchers to design and develop newer environmental friendly methods of synthesis of new or existing catalysts and/ or reagents [1, 2]. In this context,it may be emphasized that the interest in the environmental friendly synthesis of organic [3, 4] and inorganic tribromides [5, 6] has increased due to their versatile utility in organic transformations. In spite of the availability of large numbers of methodologies,most of them are not favorable due to the use of detrimental reagents (like liquid Br2) for the synthesis of tribromides. However,it is better to use environmental friendly oxidizing agents for the conversion of bromide to tribromide to achieve the synthesis of QTBs. Examples of such oxidants include oxone [7],persulphate [8],KMnO4 [9], and CAN [10]. Over the last few years,our group has been involved in the development of synthetic protocols for economical,novel and environmentally safe reagents,such as organic ammonium tribromides (OATBs) [11, 12] and catalysts [13] for important organic reactions. We have already reported the ammonium persulphate mediated synthesis of OATBs [8]. Taking cue from this,the present paper reports a new method of synthesis of PEG KBr3 using ammonium persulphate as oxidant for the conversion of Br- to Br3-. It should be noted here that potassium tribromide (KBr3) isan efficient,cheapandenvironmentally benignreagent.The application of this reagent in organic transformation reactions has not been very successful because KBr3 is unstable at room temperature. It has been observed that polyethylene glycol (PEG) acts as a good host and captures the K+ cation and thus,provides maximum stability to the reagent. Similar host-guest chemistry can be observed in the case of {[K 18-Crown-6]Br3} [14] which on recrystallization form red crystals. {[K 18-Crown-6]Br3} was also used in the bromination of activated aromatic compounds. The advantage of [{K PEG}+Br3-] over {[K 18-Crown-6]Br3} is that PEGis less expensive and the conversion of bromide to tribromide is not achieved by the addition of rather harmful liquid bromine. Moreover,the application of PEG.KBr3 has not been well explored in organic transformations other than bromination reactions. In this article we wish to report a new environmentally benign method of synthesis of [{KPEG}+Br3-] andits applicationas reagent in acylation and bromination reactions.
All the commercial chemicals are of analytical grade and used without further purification. The completion of the reaction was monitored by TLC. The synthesized tribromide was characterized with UV-vis and FT-IR spectroscopy. X-ray diffraction (XRD) analysis was also performed with diffractometer system-XPERT-PRO equipped with Cu Kα radiation. IR spectra were recorded on KBr with MAGNA 550 FTIR spectrometer. 1H NMR spectra were recorded in DMSO-d6/ CDCl3 on a Bruker Ultra Shield 400 plus spectrometer.
PEG·KBr3 was synthesized by mixing 1 mmol of PEG-4000 (4.0 g) to the solution of 3 mmol of KBr (0.36 g) in 15 mL of 2 mol/L H2SO4 and the mixture was stirred for ca. one hour. To the resulting solution,ammonium persulphate (3 mmol,0.68 g) solution was added and stirred for another 30 min. An orange-red viscous liquid of [{K PEG}+Br3-] appeared which was extracted with Et2O and dried under vacuum (yield 81%). The formation of tribromide (Scheme 1) was confirmed by electronic absorption spectroscopy (Fig. S1 in Supporting information) and FT-IR technique. An intense band observed at 274 nm is characteristic for tribromide anion (Br3-) [15]. The IR spectrum of PEG KBr3 (Fig. S2 in Supporting information) exhibits characteristic (Br3) bands at 52 (v1) and 189 (v2) cm-1 for bending and asymmetric stretching,respectively[16]. The XRD pattern of PEG KBr3 at room temperature indicates the formation of the crystalline structure. The X-ray peaks in the range of 2θ (10°< 2θ< 60°) show some weak low-angle peaks and one high angle peak at 27°. The XRD pattern of PEG (Fig. S3 in Supporting information) shows two sharp peaks at 19.3° and 23.5° which are not observed in the XRD pattern of PEG KBr3,and consistent with literature information [17, 18]. The disappearance of peaks suggests that PEG strongly interacts with KBr3 [19]. The broad peak appearing at 22° (d = 4 Å ) is a result of inter-chain interactions from London dispersion forces [20, 21]. Much information could not be inferred (resolved) regarding the packing and lattice structure of the compound due to lack of literature on the powder XRD pattern of this compound.
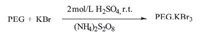
|
Download:
|
| Scheme 1. Synthesis of PEG·KBr3. | |
A representative acylation reaction was conducted by adding PEG·KBr3 (1 mmol,4.3 g) to the stirred reaction mixture of alcohol (1 mmol) and acetic acid (5 mL). The mixture was refluxed for ca. half an hour with the progress of the reaction monitored by TLC (10% ethyl acetate/hexane). After completion of the reaction,the entire mixture was poured into a saturated solution of NaHCO3 (20 mL). The product was extracted with 5 mL of ethyl acetate and dried with anhydrous sodium sulphate and evaporated under vacuum to obtain the pure product.
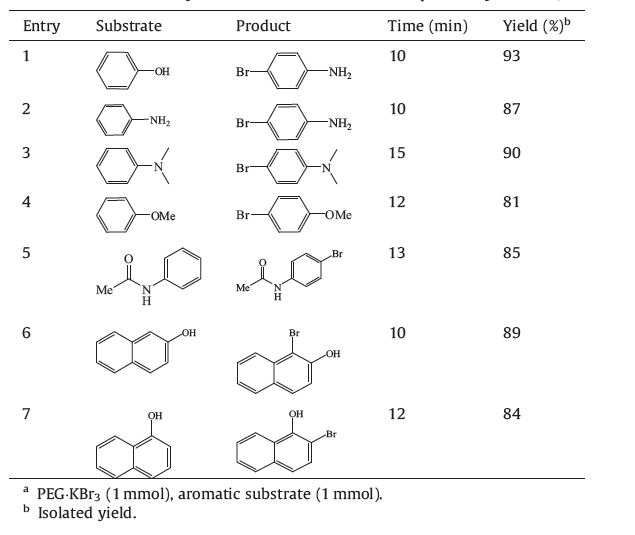
The bromination reactions were carried out in solvent free manner. In a typical reaction,PEG·KBr3 (1 mmol,4.3 g) was added to the aromatic substrate (1 mmol) in a mortar and was ground for the desired reaction time. The progress of the reaction was monitored by TLC (10% ethyl acetate/hexane). After completion of the reaction,the product was extracted with ethyl acetate and evaporated under vacuum to obtain the pure brominated product. The products were characterized by IR and NMR spectra (see Supporting information).
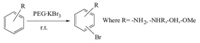
The PEG supported tribromide is a viscous liquid which is found to be highly stable and could be stored at room temperature without significant decomposition. To study the utility of PEG·KBr3,acylation and bromination reactions were conducted with some chosen organic substrates. A representative bromination is shown in Scheme 2. The plausible mechanism of acylation by PEG·KBr3 is depicted in Scheme 3. Fundamentally,PEG·KBr3 liberates Br2 which in turn forms HBr in the presence of acetic acid. Then HBr reacts with acetic acid and alcohols to generate the pure products (Scheme 3). Products were further characterized by comparing their melting point and boiling point with authentic pure samples [22]. The results have been summarized in Tables 1 and 2.
|
Download:
|
| Scheme 2. Representative bromination of aromatic substrate by PEG·KBr3. | |
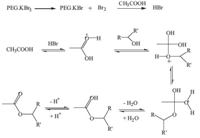
|
Download:
|
| Scheme 3. Plausible mechanism of acylation of alcohols mediated by PEG·KBr3. | |
| Table 1 Acylation of selectively chosen alcohols promoted by PEG·KBr3.a |
| Table 2 Bromination of selectively chosen activated aromatic compounds by PEG·KBr3.a |
The present protocol emphasizes the development of economic and environmentally safe synthesis of polyethylene glycol supported potassium tribromide and its useful application in the acylation of alcohols and the bromination of aromatic substrates. The advantages of this reagent are stability,high efficiency,reusability and non-hazardous nature. In spite of the availability of numerous reagents in the literature for the above mentioned organic reactions,the advantages represented herein will serve as an alternative protocol for acylation of alcohols and bromination of organic substrates.
S.S.D. acknowledges the Department of Science and Technology, DST,New Delhi,India,for financial assistance received through a SERC fast track project (No. SR/FTP/CS-100/2007).
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.05.036.
| [1] | P.T. Anastas, J.C. Warner, Green Chemistry: Theory and practice, Oxford University Press, New York, 1998. |
| [2] | S. Wenda, S. Illner, A. Mell, et al., Industrial biotechnology—the future of green chemistry, Green Chem. 13 (2011) 3007-3047. |
| [3] | C. Chiappe, E. Leandri, D. Pieraccini, Highly efficient bromination of aromatic compounds using 3-methylimidazolium tribromides as reagent/solvent, J. Chem. Soc. Chem. Commun. 45 (2004) 2536-2537. |
| [4] | A.D. Jordan, C. Luo, A.B. Reitz, Efficient conversion of substituted aryl thioureas to 2-aminobenzothiazoles using benzyltrimethyl ammonium tribromides, J. Org. Chem. 68 (2003) 8693-8696. |
| [5] | K. Ma, S. Li, R.G.Weiss, Stereoselective bromination reactions using tridecylmethylphosphonium tribromide in a "Stacked" reactor, Org. Lett. 10 (2008) 4155-4158. |
| [6] | A.R. Hajipour, S.A. Pourmousavi, A.E. Ruoho, Benzyltriphenylphosphonium tribromide: a mild, regenerable and efficient reagent for the deprotection of dithioacetals, J. Sulfur Chem. 25 (2004) 401-405. |
| [7] | V. Kavala, S. Naik, B.K. Patel, A new recyclable ditribromide reagent for efficient bromination under solvent free condition, J. Org. Chem. 70 (2005) 4267-4271. |
| [8] | M. Dey, S.S. Dhar, M. Kalita, Novel methods of synthesis of quaternary ammonium tribromides and investigation of catalytic role of benzyltrimethyl ammonium tribromide in oxidation alcohols to carbonyl compounds, Synth. Commun. 43 (2013) 1734-1742. |
| [9] | M. Dey, S.S. Dhar, Synthesis of quaternary tribromides: a novel green approach, Green Chem. Lett. Rev. 5 (2012) 639-642. |
| [10] | R. Borah, A.J. Thakur, Green synthesis of tetraalkylammonium tribromide using cerium (IV) ammonium nitrate (CAN) as oxidant, Synth. Commun. 37 (2007) 933-939. |
| [11] | U. Bora, M.K. Chaudhuri, S.S. Dhar, et al., Peroxometal-mediated environmentally favourable route to brominating agents and protocol for the bromination of organics, Pure Appl. Chem. 73 (2001) 93-102. |
| [12] | M. K. Chaudhuri, U. Bora, S.S. Dhar, et al., Process of preparing quaternary ammonium tribromides, US Patent 7005548 (2006). |
| [13] | M. Dey, K. Deb, S.S. Dhar, VO(acac)2 catalysed condensation of o-phenylenediamine with aromatic carboxylic acids/aldehydes under microwave radiation affording benzimidazoles, Chin. Chem. Lett. 22 (2011) 296-299. |
| [14] | M.A. Zolfigol, G. Chehardoli, S. Salehzadeh, et al., {[K 18-Crown-6]Br3}n: a unique tribromide-type and columnar nanotube-like structure for the oxidative coupling of thiols and bromination of some aromatic compounds, Tetrahedron Lett. 48 (2007) 7969-7973. |
| [15] | M. Hossein, K. Zahra, Synthesis and application of poly (diallyldimethylammonium tribromide) as a novel polymeric brominating agent, Chin. J. Chem. 28 (2010) 2221-2225. |
| [16] | K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed., John Wiley & Sons Inc., New York, 1997p. 169. |
| [17] | T. Ozeki, H. Yuasa, Y. Kanaya, Application of the solid dispersion method to the controlled release of medicine. IX. Difference in the release of flurbiprofen from solid dispersions with poly(ethylene oxide) and hydroxypropylcellulose and the interaction between medicine and polymers, Int. J. Pharm. 155 (1997) 209-217. |
| [18] | K. Shameli, M.B. Ahmad, S.D. Jazayeri, Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method, Int. J. Mol. Sci. 13 (2012) 6639-6650. |
| [19] | E. Kang, J. Robinson, K. Park, et al., Paclitaxel distribution in poly(ethylene glycol)/poly(lactide-co-glycolic acid) blends and its release visualized by coherent anti-Stokes Raman scattering microscopy, J. Control. Release 122 (2007) 261-268. |
| [20] | V. Meisalo, O. Inkinen, An X-ray diffraction analysis of potassium bromide, Acta Crystallogr. 22 (1967) 58-65. |
| [21] | H.B. Watson, The reaction of bromine with aliphatic acids. Catalytic effect of acyl halides, J. Chem. Soc. Trans. 127 (1925) 2067-2082. |
| [22] | J. Buckinghum, S.M. Donaghy, Dictionary of Organic Compounds, 6th ed., Chapman & Hall, London, 1982. |