b Department of Chemistry, Tianjin Key Laboratory of Metal and Molecule-Based Material Chemistry and Key Laboratory of Advanced Energy Materials Chemistry (MOE), Nankai University, Tianjin 300071, China;
c State Key of Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, China
As one of the vast family of porous materials,metal-organic frameworks (MOFs) are rapidly expanded for their intriguing structural architectures and their potential applications in catalysis,drug delivery,gas sorption and storage,separation, and sensor [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. Recently,increasing attention has been paid to the development of synthetic strategies for MOFs with novel structures and special properties. To date,several effective synthetic strategies have been developed. One of feasible approaches is employing large size organic species as ligands [13, 14, 15]. The second powerful strategy is utilizingmetal-organic polyhedra (MOP) as the secondary building units [16, 17]. Recently,template-directing method has been proved to be an effective strategy in the synthesis of MOFs [18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42]. Since then,various templates such as cations [21, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40],anions [18, 19, 20, 21, 41, 42],and neutral groups [22, 23, 24, 25, 26, 27, 28],have been employed. Typical templates include polyoxometalates [18, 19, 20, 21], water clusters [21, 22, 23, 24, 25, 26, 27, 28],and organic species [29, 30, 31]. We newly introduce the transition metal complexes,which have been commonly used in the synthesis of metal phosphates and germanates [43, 44, 45, 46, 47],as template in the synthesis of MOFs systems. And a series of metal oxalates have been synthesized by using transition metal complexes as template [33, 34, 35, 36, 37, 38, 39, 40],which approved that it is an effective strategy to obtainMOFs with large channel open frameworks. However,it still remains a great challenge for constructing MOFs with open frameworks by the template-directing method since such results are a tiny part among all of the explored MOFs.
As one contemporary aspect of template-directing method, co-templating effect has been observed in some structures which were obtained in the presence of more than one template [45, 46, 47, 48, 49, 50, 51]. For example,Pan and Yu have constructed two novel germanates (JLG-4 and 5) with 12-ring tubular structures by using Ni(1,2-pda)32+/3-amino-1-propanol and (H2O)16 cluster/2- methylpiperazine as co-template,respectively [45, 46]. However, the application of co-templating strategy in the synthesis of MOFs is rare. The remarkably example is [Co4(dpdo)12] [H(H2O)27(CH3CN)12][PW12O40]3,which is co-templated by PW12O403- and H+(H2O)27 [21]. Therefore,employing more than one species as co-templates may result in the MOFs with novel and interesting structures.
Accordingly,to further understand the influence of template in MOFs,our continuing research interest has been focused on the template-directing synthetic strategy in microporous open-framework metal oxalates by Co(III) complexes as template [38, 39, 40]. In this work,by using Co(NH3)63+ cations as template,we prepared a novel three-dimensional (3D) open-framework cadmium oxalate, [Co(NH3)6]2[Cd8(C2O4)11(H2O)4]·8H2O (denoted HNU-1). Its 3D anionic framework with 12-ring channels displays a sqp topology with dinuclear Cd(II) clusters (Cd2 clusters) acting as nodes. The Co(NH3)63+ cations and unusual hydrogen-bonded (H2O)4 clusters are found in the 12-ring channels with an alternative arrangement. It is believed that the (H2O)4 clusters play a co-templating role in the crystallization of HNU-1.
All chemicals purchased were of reagent grade and used without further purification. Thermogravimetric analyses (TGA) were performed under air condition from room temperature to 800 ℃ with a heating rate of 10 ℃/min on Perkin-Elmer TGA-7 thermogravimetric analyzer. Element analyses (C,H and N) were carried out with a Perkin-Elmer 2400 elemental analyzer. ICP analysis was carried out on a IRIS Advantage ICP instrument. The infrared (IR) spectra were recorded from 400 cm-1 to 4000 cm-1 on a Nicolet Impact 410 FTIR spectrometer by using KBr pellets. Xray powder diffraction (XRD) data for HNU-1 was collected on a Rigaku X-ray diffractometer using Cu-Kα radiation (λ= 1.5418Å ).
HNU-1 was prepared under hydrothermal condition. A mixture of 0.268 g Na2C2O4,0.308 g Cd(NO3)2·4H2O,0.1 g Co(NH3)6·Cl3,and 10 mL H2O was added to 25 mL Teflon-lined reactor under autogenous pressure at 100 ℃ for 4 days. The autoclave was then cooled to room temperature. Yellow rod-shaped crystals of HNU-1 were obtained. After isolated,the crystals of HNU-1 were washed with distilled water,and dried in air,with the yield of 50% based on Co(NH3)6·Cl3. Anal. Calcd. (wt%) for C22H60 N12O56Cd8Co2: C, 10.98; H,2.51; N,6.99; Co,4.90; Cd,37.38. Found (wt%): C,11.25; H,2.71; N,7.04; Co,4.87; Cd,37.55. IR data (KBr,cm-1,Fig. S1 in Supporting information): 3134 (s),2360 (w),1609 (s),1401 (s), 1312 (m),1047 (w),953 (w),880 (w),791 (m),649 (w),486 (w).
The suitable yellow rod-shaped single crystal with the dimensions of 0.15 mm × 0.15 mm × 0.1 mm was selected for single-crystal X-ray diffraction analysis for HNU-1. X-ray singlecrystal diffraction data of HNU-1 were collected on a Rigaku SCXmini diffractometer in the range of 3.08-27.49° at the temperature of (293 ± 2) K using graphite-monochromated Mo Kαradiation λ= 0.71073Å ). The number of collected reflections and independent reflections were 15,182 and 3480 for HNU-1. The program SAINT [52] was used for integration of the diffraction profiles. The structure was used for integration of the diffraction profiles. The structure was solved by direct methods using the SHELXS programof the SHELXTL package and refined by full-matrix least-squares methods with SHELXL [53]. The Cd and Co atoms were located first from the E-maps. Then O,C,and N atoms were found in successive difference Fourier maps. All the non-hydrogen atoms were refined anisotropically,which gave an empirical framework formula of [Co(NH3)6]2[Cd8(C2O4)11(H2O)4]·8H2O. CCDC-898581 contains the supplementary crystallographic data for this paper. Such data can be obtained free of charge at http://www.ccdc.cam.ac.uk/conts/retrieving. html. Experimental details for crystal structure determinations of HNU-1 are listed in Table 1. The selected bond lengths and angles of HNU-1 are given in Table S1 in Supporting information.
| Table 1 Crystallographic data for HNU-1. |
Single-crystal X-ray diffraction analysis indicates that HNU-1 crystallizes in the monoclinic space group C2/c with a= 11.126(2)Å ,b= 17.361(4)Å ,c= 16.119(3)Å ,b= 102.40(3)8, and Z = 8 [53]. It exhibits a 3D open-framework structure consisting of macroanionic [Cd8(C2O4)11(H2O)4]n6n- framework, which is built from Cd2 clusters. The negative charges of the framework are compensated by Co(NH3)63+ cations,which alternated by a unusual hydrogen-bonded (H2O)4 clusters to fill the 12-ring channels as co-template.
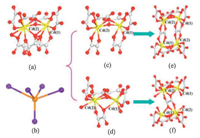
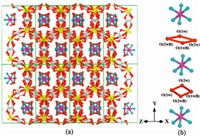
As shown in Fig. 1,the Cd2 cluster is composed of a pair of Cd(1) and Cd(2) atoms,which contains twokinds of possiblemodes with the ratio of 50:50 for the intramural oxalate ligands position disordered. For type I,Cd(1) and Cd(2) atoms have similar coordinatedmodes,both of themare seven-coordinated by seven O atoms from three bidentate oxalate units and one monodentate oxalate unit,as shown in Fig. 1c. For type II,as shown in Fig. 1d,it contains two Cd atoms,five oxalate units,and two coordinated water molecules. Cd(1) atom is eight-coordinated by eight O atoms from four oxalate units (three bidentate units and one monodentate unit) and a coordinatedwater molecule. Cd(2) atom is also surrounded by four oxalate units and a coordinated water molecule,however,it presents a seven-coordinatedmode for two of oxalate units are bidentate units and the other two are monodentate units. Both modes of Cd2 clusters linked five adjacent Cd2 clusters by oxalate units,which could be regarded as a distorted 5-connected building unit,as shown in Fig. 1b. Two such Cd2 clusters with the same type link each other by oxalate ligands to form 8-connected Cd4(C2O4)10 motif (Fig. 1e) for type I, and 8-connected Cd4(C2O4)9(H2O)4 motif (Fig. 1f) for type II, respectively.
|
Download:
|
| Fig. 1. (a) The Cd2 cluster with parts of oxalate ligands position disordered; (b) five-connected building units; (c) and (d) are the Cd2 clusters with two kinds of possible modes;(e) and (f) are eight-connected motifs linked by two Cd2 clusters with same type,respectively. | |
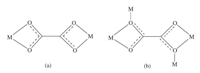
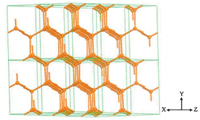
The Cd2 clusters are further linked each other to form a 3D [Cd8(C2O4)11(H2O)4]n6n- macroanionic framework by sharing oxalate units. Although two kinds of oxalate units,4-connected and bis-bidentate oxalate units (illustrated in Scheme 1),have been found around Cd2 clusters,both of them linked with two neighborhood Cd2 clusters could be regarded as 2-connected linker. After simplification,the 3D framework presents a uninodal 5-connected sqp network with the short point symbol of {44.64} (td10 = 1366; Fig. 2) [54]. Interestingly,this 3D framework can also be simplicated to a uninodal 8-connected bcu network with the short point symbol of {424.64} (td10 = 2331),based on Cd4(C2O4)10/ Cd4(C2O4)9(H2O)4 motifes as the nodes [51].
|
Download:
|
| Scheme 1. The coordination modes of oxalate units in HNU-1. | |
|
Download:
|
| Fig. 1. The 3D 5-connected sqp topological network of HNU-1 based on Cd2 cluster. | |
|
Download:
|
| Fig. 3. (a) Structure of HNU-1 viewed along [1 0 1] direction; (b) with the hydrogen-bonded (H2O)4 clusters alternately arranged with Co(NH3)63+ cations are encapsulated inside the 12-ring channels as the co-template. | |
The TG analysis of HNU-1 (Fig. S7 in Supporting information) shows two weight loss steps between 20 ℃ and 700 ℃. The first weight loss of 5.9% from 20 ℃ to 150 ℃ is attributed to the loss of free water molecules. The second weight loss of 44.2% between 200 ℃ and 370 ℃ is caused by the decomposition of the metal complexes and the collapse of the framework.
Up to now,series of 3D oxalate compounds templated by metal complexes have been reported,all the open-frameworks of these compounds present large/extra large channels as shown in Table 2. [Co(dien)2][Cd4(H2O)2(ox)7]0.54.5H2O presents elliptical 20-ring channel templated by pairs of Co(dien)23+ [39]. [MII(bpy)3][M'II2(ox)3] and [MII(bpy)3][M'IM''III(ox)3] show 18-ring channel by using MII(bpy)33+ as template [33, 35],NKB-1 exhibits elliptical 16-ring channel filled by disorder Co(en)33+ complexes [38], [Co(dien)2][NaCo2(ox)4]0.5·H2O presents 14-ring channel by employing Co(dien)23+ as template [40],and HNU-1 presents 12-ring channel by using Co(NH3)63+ as template. Further research on the relationship of pores and metal complexes,it is shown that the pore rings of these compounds decrease in turn from 20-,18-, 16-,14- to 12-rings,in accordance with the sizes of corresponding complexes from pairs of Co(dien)23+,MII(bpy)33+,1.2 Co(en)33+, Co(dien)23+ to Co(NH3)63+. Therefore,it is very important for the sizes of templates that they could direct to form metal-organic frameworks with the corresponding sizes of pores.
| Table 2 3D oxlate compounds templated by metal complexes. |
In summary,a new 3D cadmium oxalate HNU-1 is hydrothermally synthesized in the presence of Co(NH3)6Cl3. Its 3D openframework presents 5-connected sqp topology based on Cd2 cluster. Notably,an unusual hydrogen-bonded (H2O)4 cluster is found,and such water clusters are alternately arranged with Co(NH3)63+ cations to be encapsulated inside the 12-ring channels as co-template. Compared with other oxalate compounds templated by metal complexes,it is suggested that it should be an effective strategy to obtain MOFs with specifically pores by control the sizes of the templates,such as metal complexes.
This work was supported by the National Natural Science Foundation of China (No. 21101047),the Program for New Century Excellent Talents in University (No. NCET-11-0929),the Natural Science Foundation of Hainan Province (No. 211010),the Priming Scientific Research Foundation of Hainan University (No. kyqd1051).
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.06.025.
| [1] | B. Chen, M. Eddaoudi, S.T. Hyde, M. O'Keeffe, O.M. Yaghi, Interwoven metal-organic framework on a periodic minimal surface with extra-large pores, Science 291 (2001) 1021-1023. |
| [2] | G. Férey, C. Mellot-Draznieks, C. Serre, et al., A chromium terephthalate-based solid with unusually large pore volumes and surface area, Science 309 (2005) 2040-2042. |
| [3] | Y.F. Zeng, X. Hu, F.C. Liu, X.H. Bu, Azido-mediated systems showing different magnetic behaviors, Chem. Soc. Rev. 38 (2009) 469-480. |
| [4] | X.M. Chen, M.L. Tong, A new bridge between coordination chemistry and organic synthetic chemistry, Acc. Chem. Res. 40 (2007) 162-170. |
| [5] | S.L. Qiu, G.S. Zhu, Molecular engineering for synthesizing novel structures of metal-organic frameworks with multifunctional properties, Coord. Chem. Rev. 253 (2009) 2891-2911. |
| [6] | B. Moulton, M.J. Zaworotko, From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids, Chem. Rev. 101 (2011) 1629-1658. |
| [7] | Y.K. Park, S.B. Choi, H. Kim, et al., Crystal structure and guest uptake of a mesoporous metal-organic framework containing cages of 3.9 and 4.7 nm in diameter, Angew. Chem. Int. Ed. 46 (2007) 8230-8233. |
| [8] | J. An, S.J. Geib, N.L. Rosi, Cation-triggered drug release from a porous zinc-adeninate metal-organic framework, J. Am. Chem. Soc. 131 (2009) 8376-8377. |
| [9] | J.R. Li, Y. Tao, Q. Yu, et al., Selective gas sorption and unique structural topology of a highly stable guest-free zeolite-type MOF material with N-rich chiral open channels, Chem. Eur. J. 14 (2008) 2771-2776. |
| [10] | Q.R. Fang, G.S. Zhu, Z. Jin, et al., Mesoporous metal-organic framework with rare etb topology for hydrogen storage and dye assembly, Angew. Chem. Int. Ed. 46 (2007) 6638-6642. |
| [11] | Q.H. Pan, Q. Chen, Z. Chang, et al., A 3D lead(Ⅱ) coordination polymer containing helical chains with rare ecl topology, Chin. J. Inorg. Chem. 26 (2010) 2299-2302. |
| [12] | K.H. He, Y.W. Li, Y.Q. Chen, Z. Chang, A new 8-connected self-penetrating metal-organic framework based on dinuclear cadium clusters as secondary building units, Chin. Chem. Lett. 24 (2013) 691-694. |
| [13] | M. Eddaoudi, J. Kim, N. Rosi, et al., Systematic design of pore size and functionality in isoreticular metal-organic frameworks and application in methane storage, Science 295 (2002) 469-472. |
| [14] | O.K. Farha, C.D. Malliakas, M.G. Kanatzidis, J.T. Hupp, Control over catenation in metal organic frameworks via rational design of the organic building block, J. Am. Chem. Soc. 132 (2009) 950-952. |
| [15] | X.S. Wang, S.Q. Ma, D.F. Sun, S. Parkin, H.C. Zhou, A mesoporous metal-organic framework with permanent porosity, J. Am. Chem. Soc. 128 (2006) 16474-16475. |
| [16] | F. Nouar, J.F. Eubank, T. Bousquet, et al., Tunable zeolite-like metal-organic frameworks (ZMOFs): lithium and magnesium ion exchange and H2-(rho-ZMOF) interaction studies, J. Am. Chem. Soc. 130 (2008) 1833-1835. |
| [17] | J.J. Perry IV, V.Ch. Kravtsov, G.J. McManus, M.J. Zaworotko, Bottom up synthesis that does not start at the bottom: quadruple covalent cross-linking of nanoscale faceted polyhedra, J. Am. Chem. Soc. 129 (2007) 10076-10077. |
| [18] | X.Y. Zhao, D.D. Liang, S.X. Liu, et al., Two dawson-templated three-dimensional metal-organic frameworks based on oxalate-bridged binuclear cobalt(Ⅱ)/nickel(Ⅱ) SBUs and bpy linkers, Inorg. Chem. 47 (2008) 7133-7138. |
| [19] | C.Y. Sun, S.X. Liu, D.D. Liang, et al., Two dawson-templated three-dimensional metal-organic frameworks based on oxalate-bridged binuclear cobalt(Ⅱ)/Nickel(Ⅱ) SBUs and bpy linkers, J. Am. Chem. Soc. 131 (2009) 1883-1888. |
| [20] | X.L. Wang, H.L. Hu, A.X. Tian, H.Y. Lin, J. Li, Application of tetrazole-functionalized thioethers with different spacer lengths in the self-assembly of polyoxometalatebased hybrid compounds, Inorg. Chem. 49 (2010) 10299-10306. |
| [21] | M.L. Wei, C. He, W.J. Hua, et al., A large protonated water cluster H+(H2O)27 in a 3D metal organic framework, J. Am. Chem. Soc. 128 (2006) 13318-13319. |
| [22] | H.H. Song, B.Q. Ma, A well-resolved discrete dodecameric water cluster in a metal-organic complex, CrystEngComm 9 (2007) 625-627. |
| [23] | L.Y. Wang, Y. Yang, K. Liu, B.L. Li, Y. Zhang, A new "opened-cube" (H2O)10 cluster and undulated water chain in porous metal organic frameworks, Cryst. Growth Des. 8 (2008) 3902-3904. |
| [24] | L.B. Sun, Y. Li, Z.Q. Liang, J.H. Yu, R.R. Xu, Structures and properties of lanthanide metal-organic frameworks based on a 1,2,3-triazole-containing tetracarboxylate ligand, Dalton Trans. 41 (2012) 12790-12796. |
| [25] | L. Infantes, J. Chisholm, S. Motherwell, Extended motifs from water and chemical functional groups in organic molecular crystals, CrystEngComm 5 (2003) 480-486 (and reference therein). |
| [26] | M. Mascal, L. Infantes, J. Chisholm, Water oligomers in crystal hydrates-what's news and what isn't? Angew. Chem. Int. Ed. 45 (2006) 32-36 (and reference therein). |
| [27] | Q. Yu, Y.F. Zeng, J.P. Zhao, et al., Three-dimensional porous metal organic frameworks exhibiting metamagnetic behaviors: synthesis, structure, adsorption, and magnetic properties, Inorg. Chem. 49 (2010) 4301-4306. |
| [28] | Y. Wang, T. Okamura, W.Y. Sun, N. Ueyama, Large (H2O)56(OH)6 and (H2O)20 clusters inside a nanometer-sized M6L8 cage constructed by five-coordinated copper(Ⅲ) and flexible carboxamide-containing tripodal ligand, Cryst. Growth. Des. 8 (2008) 802-804. |
| [29] | Q.R. Fang, G.S. Zhu, M. Xue, et al., Amine-templated assembly of metal-organic frameworks with attractive topologies, Cryst. Growth Des. 8 (2007) 319-329. |
| [30] | Y.L. Liu, V.C.h. Kravtsov, M. Eddaoudi, Template-directed assembly of zeolite-like metal-organic frameworks (ZMOFs): a usf-ZMOF with an unprecedented zeolite topology, Angew. Chem. Int. Ed. 47 (2008) 8446-8449. |
| [31] | J.H. He, J.H. Yu, Y.T. Zhang, Q.H. Pan, R.R. Xu, Synthesis, structure, and luminescent property of a heterometallic metal organic framework constructed from rodshaped secondary building blocks, Inorg. Chem. 44 (2005) 9279-9282. |
| [32] | M. Dan, C.N.R. Rao, A building-up process in open-framework metal carboxylates that involves a progressive increase in dimensionality, Angew. Chem. Int. Ed. 45 (2006) 281-285. |
| [33] | H. Okawa, A. Shigematsu, M. Sadakiyo, et al., Oxalate-bridged bimetallic complexes {NH(prol)3}[MCr(ox)3] (M=MnⅡ, FeⅡ, CoⅡ; NH(prol)3+ = tri(3-hydroxypropyl) ammonium) exhibiting coexistent ferromagnetism and proton conduction, J. Am. Chem. Soc. 131 (2009) 13516-13522. |
| [34] | Y.F. Zeng, X. Hu, J.P. Zhao, et al., Partial substitution of hydroxyl by azide: an unprecedented 2D azido-copper-hydroxyl compound with a [Cu24] macrocycle in the presence of [Cu(H2O)6]2+, Chem. Eur. J. 14 (2008) 7127-7130. |
| [35] | R.Q. Zhong, R.Q. Zou, D.S. Pandey, T. Kiyobayashi, Q. Xu, A novel 3D microporous metal-organic framework of cadmium(Ⅱ) oxalate with diamondoid network, Inorg. Chem. Commun. 11 (2008) 951-953. |
| [36] | S. Decurtins, H.W. Schmalle, P. Schneuwly, J. Ensling, P. Guetlich, A concept for the synthesis of 3-dimensional homo-and mimetallic oxalate-bridged networks[M2(ox)3]n. Structural, moessbauer, and magnetic studies in the field of molecular-based magnets, J. Am. Chem. Soc. 116 (1994) 9521-9528. |
| [37] | Z.X. Chen, H.Y. Yang, M.L. Deng, et al., Metal complexes as templates: syntheses, structures, and luminescent properties of two zinc phosphonocarboxylates with ABW-zeolite topology, Dalton Trans. 41 (2012) 4079-4083. |
| [38] | Q.H. Pan, J.Y. Li, Q. Chen, et al., |Co(en)3|1/3[In(ox)2]·3.5H2O: a zeolitic metal-organic framework templated by Co(en)3Cl3, Microporous Mesoporous Mater. 132 (2010) 453-457. |
| [39] | Q.H. Pan, Q. Chen, W.C. Song, T.L. Hu, X.H. Bu, Template-directed synthesis of three new open-framework metal(Ⅱ) oxalates using Co(Ⅲ) complex as template, CrystEngComm 12 (2010) 4198-4204. |
| [40] | Q.H. Pan, Q. Chen, Y.D. Han, T.L. Hu, X.H. Bu, Template-directed synthesis of a novel oxalate compound [Co(dien)2][NaCo2(C2O4)4] using Co(Ⅲ) complex as template, Chem. J. Chin. Univ. 32 (2011) 527-531. |
| [41] | Z. Chang, A.S. Zhang, T.L. Hu, X.H. Bu, ZnⅡ coordination poylmers based on 2,3,6,7-anthracenetetracarboxylic acid: synthesis, structures, and luminescence properties, Cryst. Growth Des. 9 (2009) 4840-4846. |
| [42] | Q.H. Pan, H. Ma, Z.X. Li, T.L. Hu, Anions behaviors for the dimensionalities of coordination polymers based on poly(imidazole) ligands, J. Mol. Struct. 1011 (2012) 134-139. |
| [43] | Y. Wang, J.H. Yu, Y. Li, Z. Shi, R.R. Xu, Chirality transfer from guest chiral metal complexes to inorganic framework: the role of hydrogen bonding, Chem. Eur. J. 9 (2003) 5048-5055. |
| [44] | Y. Wang, J.H. Yu, M. Guo, R.R. Xu, [{Zn2(HPO4)4}{Co(dien)2}]·H3O: a zinc phosphate with multidirectional intersecting helical channels, Angew. Chem. Int. Ed. 42 (2003) 4089-4092. |
| [45] | Q.H. Pan, J.Y. Li, X.Y. Ren, et al., [Ni(1,2-PDA)3]2(HOCH2CH2CH2NH3)3(H3O)2[Ge7O14X3]3 (X = F, OH): a new 1D germanate with 12-ring hexagonal tubular channels, Chem. Mater. 20 (2008) 370-372. |
| [46] | Q.H. Pan, J.Y. Li, K.E. Christensen, et al., A germanate built from a 68126 cavity cotemplated by an (H2O)16 cluster and 2-methylpiperazine, Angew. Chem. Int. Ed. 47 (2008) 7868-7871. |
| [47] | Q.H. Pan, X.Y. Ren, Y. Xu, W.F. Yan, (C4N2H12)(NH4)2[(GeO2)3(GeO1.5F3)2]: a new layered germanate containing helical arrays of H-bond, Inorg. Chem. Commun. 14 (2011) 1842-1845. |
| [48] | J.H. Yu, R.R. Xu, Insight into the construction of open-framework aluminophosphates, Chem. Soc. Rev. 35 (2006) 593-604. |
| [49] | B. Wei, J.H. Yu, Z. Shi, S.L. Qiu, J.Y. Li, A new layered aluminophosphate[Al2P4O16][C6H22N4][C2H10N2] with 4.12-net porous sheets, J. Chem. Soc., Dalton Trans. 13 (2000) 1979-1980. |
| [50] | Y.Y. Wang, Y. Li, L. Wang, et al., ACO-Zeotype iron aluminum phosphates with variable Al/Fe ratios controlled by F- ions, Inorg. Chem. 50 (2011) 1820-1825. |
| [51] | H.Z. Xing, J.Y. Li, W.F. Yan, et al., Cotemplating ionothermal synthesis of a new open-framework aluminophosphate with unique Al/P ratio of 6/7, Chem. Mater. 20 (2008) 4179-4181. |
| [52] | Bruker AXS, SAINT Software Reference Manual, Brucker Analytical X-ray instruments Inc., Madison, WI, 1998. |
| [53] | G.M. Sheldrick,Ashort history ofSHELX, Acta Crystallogr.Sect.A64(2008)112-122. |
| [54] | E.V. Alexandrov, V.A. Blatov, A.V. Kochetkov, D.M. Proserpio, Underlying nets in three-periodic coordination polymers: topology taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database, CrystEngComm 13 (2011) 3947-3958 (and reference therein). |