1. Introduction
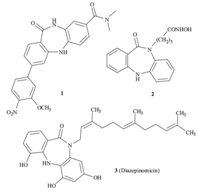
Dibenzodiazepinone and its analogs are considered as an important class of heterocyclic compounds that exhibit significant biological activities [1, 2] Previous studies on dibenzodiazepinones led not only to the discovery of drug leads,such as the Chk1 kinase inhibitor 1 [3] and the histone deacetylase inhibitors 2 [4] ,but also to the total synthesis of natural product 3 [5] isolated from marine micromonospora with potent activity against Gram-positive bacteria (Fig. 1).
|
Download:
|
| Fig. 1.Structures of natural and synthesized biologically important dibenzodiazepinones. | |
Traditional syntheses of dibenzodiazepinone derivatives rely on the preparation of amide[6]or lactam intermediates [7]or reduction of a NO2 group [8] , which resulted in multistep reactions, harsh conditions,purification of intermediates from each step,and low yields of products. Wang et al. [9] reported a coupling reaction of dibenzodiazepines from o-halobenzoic acid with diamine in chlorobenzene. However,this method gave coupling products in extremely low yield. Recently,Tsvelikhovsky and Buchwald [10] reported a novel method for preparing substituted dibenzodiazepines that included a two-step,palladium catalyzed,crosscoupling reaction and subsequent intramolecular acylation to form the products. This method relies on specific ligands for each step to achieve heterocyclic ring formation.
In the course of our studies on new methods for heterocycle synthesis,we were pleased to find that ethyl 2-bromobenzoates reacted well with o-phenylenediamine in the presence of CuI to give dibenzodiazepinones in one pot. Herein,we wished to disclose the details of our results. 2. Experimental
All 1H NMR spectra were recorded at 300 MHz or 400 MHz,and
13C NMR spectra were recorded at 100 MHz,respectively. High
resolution mass spectra were recorded on Agilent LC/MSD TOF.
Melting points were obtained on a Yanaco micrometer and all of
the temperatures were uncorrected. All reagents were used
directly as obtained commercially,unless otherwise noted.
Under an argon atmosphere,to a 50 mL sealed tube was added
o-phenylenediamine (5) (108.2 mg,1.0 mmol),CuI (19.0 mg,
0.1 mmol),K3PO4 (424.5 mg,2.0 mmol),ethylene glycol
(10.0 mL) and ethyl 2-iodobenzoate (4a) (552.1 mg,2.0 mmol).
The mixture was stirred at 100 8C for 4 h. After cooling to room
temperature,the mixture was extracted with CH2Cl2 (3 ×30 mL).
The combined organic phase of CH2Cl2 was dried over anhydrous
Na2SO4,filtered,and concentrated under reduced pressure. The
residue was purified by flash column chromatography (EtOAc/
PE = 20/80) to give 5H-dibenzo[b,e][1, 4]diazepin-11(10H)-one
(6a) as a yellow solid in 86.3% yield.
Data of the selected compounds are as follows.
5H-Dibenzo[b,e][1, 4]diazepin-11(10H)-one (6a): Mp: 250-
253 8C.1H NMR (300 MHz,DMSO-d6):
δ 9.82 (s,1H),7.82 (s,1H),7.68-7.65 (d,1H,J = 7.8 Hz),7.35-7.29 (dt,1H,J1 = 7.8 Hz,δJ2 = 1.5 Hz),7.00-6.85 (m,6H); 13C NMR (100 MHz,DMSO-d6): δ167.92,150.43,139.95,133.18,132.08,129.82,124.47,122.92, 122.76,121.26,120.69,119.76,119.04. HRMS (m/z): Calcd. for C13H11N2O: 211.0866 [M+H]+,found: 211.0870.
4-Methyl-5H-dibenzo[b,e][1, 4]diazepin-11(10H)-one (6b): Mp:
214-216 8C. 1H NMR (300 MHz,DMSO-d6): δ 9.97 (s,1H),7.51-
7.48 (d,1H,J = 7.5 Hz),7.28-7.25 (d,1H,J = 6.9 Hz),7.12-7.10 (d,
1H,J = 6.9 Hz),7.00-6.83 (m,5H),2.36 (s,3H); 13CNMR(100 MHz,
DMSO-d6): δ 168.54,148.30,140.15,134.10,131.08,129.68,
126.93,125.02,124.26,123.37,122.00,121.11,121.09,17.86.
HRMS (m/z): Calcd. for C14H13N2O: 225.1022 [M+H]+,found:
225.1022.
2-Bromo-5H-dibenzo[b,e][1, 4]diazepin-11(10H)-one (6h): Mp:
264-266 8C. 1H NMR (300 MHz,DMSO-d6): δ 9.95 (s,1H),8.03 (s,
1H),7.73 (s,1H),7.49-7.47 (d,1H,J = 8.1 Hz),6.95-6.93 (m,5H);
13C NMR (100 MHz,DMSO-d6): δ 166.47,149.62,139.16,135.66,
134.07,129.42,124.71,124.27,123.26,121.45,121.28,119.86,
111.82. HRMS (m/z): Calcd. for C13H10BrN2O: 288.9971 [M+H]+,
found: 288.9973.
N-(11-Oxo-10,11-dihydro-5H-dibenzo[b,e][1, 4]diazepin-2-yl)
acetamide (6j): Mp: 259-261 8C. 1H NMR (300 MHz,DMSO-d6): δ
9.82 (s,2H),7.82 (s,1H),7.69 (s,1H),7.59-7.56 (d,1H,J = 8.7 Hz),
6.95-6.87 (m,5H),1.98 (s,3H); 13C NMR (100 MHz,DMSO-d6): δ 167.86,167.65,145.84,140.42,132.86,129.77,124.51,122.92,
122.74,122.33,121.27,119.61,119.22,23.77. HRMS (m/z): Calcd.
for C15H14N3O2: 268.1081 [M+H]+,found: 268.1084.
3. Results and discussion
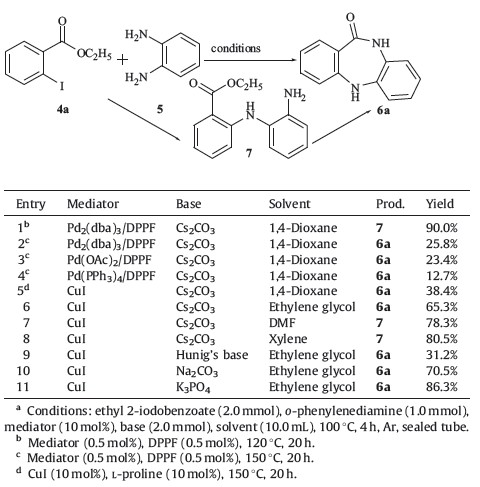
We envisioned that 7 could be formed by cross-coupling of ethyl 2-iodobenzoate 4a with o-phenylenediamine so that dibenzodiazepinones would be generated by subsequent intramolecular acylation (Table 1). To prove our hypothesis,the reaction of ethyl 2-iodobenzoate and o-phenylenediamine was carried out in the presence of Pd2(dba)3/DPPF catalyst in 1,4-dioxane at 120 8C under an argon atmosphere. Only the N-arylation product 7 was obtained in 90.0% yield (entry 1,Table 1),which indicated that the cross coupling proceeded smoothly,but failed to initiate intramolecular acylation. Subsequently,the reaction mixture was stirred at 150 8C for 20 h to promote the N-acylation cyclization,and 6a was obtained in low yield (entry 2,Table 1). Changing a range of catalysts (Pd(OAc)2/DPPF,Pd(PPh3)4/DPPF and CuI) (entries 3-5, Table 1),indicated that CuI gave higher a yield of 6a (entry 5, Table 1). Furthermore,solvents and bases were screened (entries 6-11,Table 1) and determined that ethylene glycol could promote the cyclization and a higher yield of product 6a can be obtained. Weak inorganic bases are more favorable than organic bases (entry 9,Table 1). Highest yield of 6a was achieved using a combination of CuI/K3PO4/ethylene glycol at 100 8C for 4 h (entry 11,Table 1).
| Table 1 Optimization of reaction conditions.a |
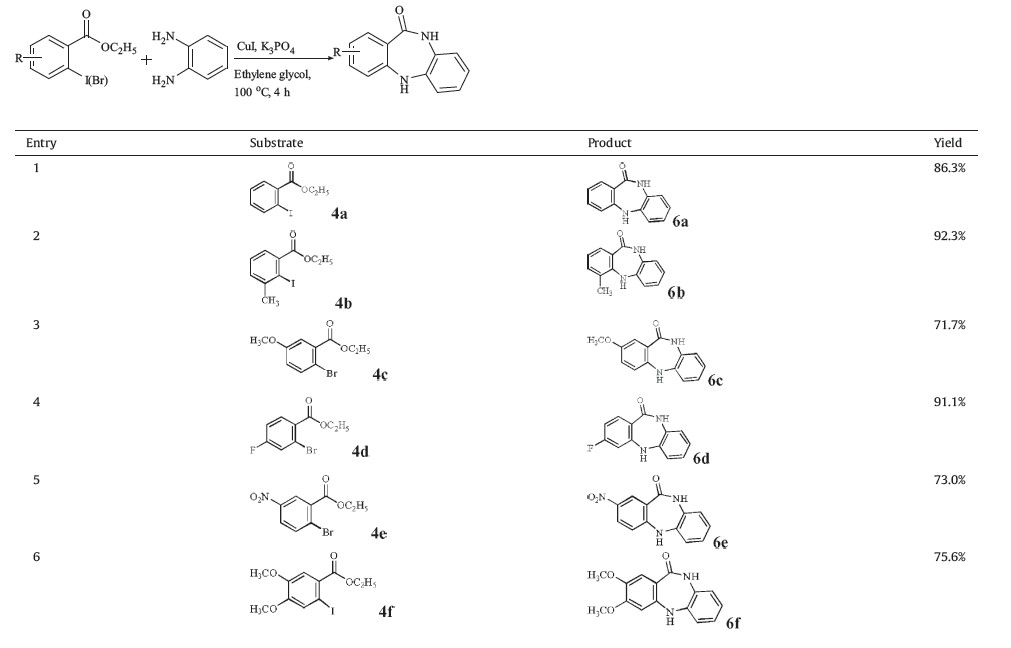
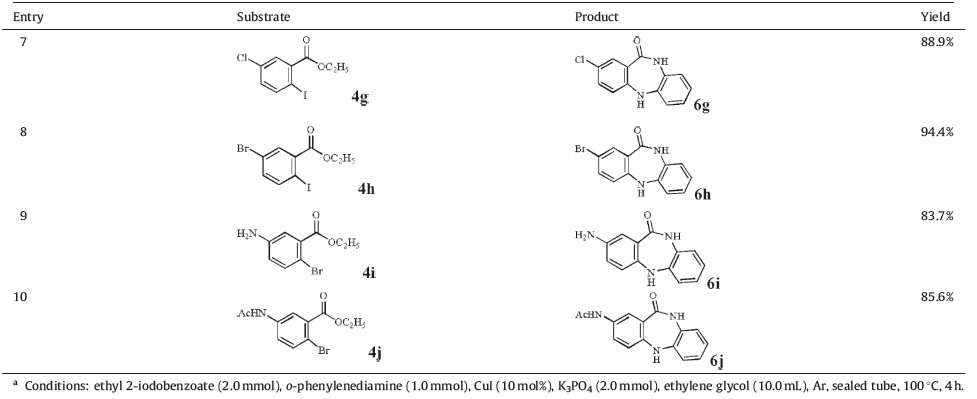
With the optimized conditions in hand,we investigated more substrates to evaluate the tolerance of this system and found that a wide range of dibenzodiazepinone derivatives could be obtained. Both ethyl 2-iodo and 2-bromobenzoate work well with this protocol. The substituents on the phenyl ring do not have an impact on the reaction and give fair to good yields. Sterically hindered ethyl 2-iodobenzoate analogs also produced successful derivatives in good yield (entry 2,Table 2). It is worth noting,that additional halogens,such as substituted chloro and bromo,as well as unprotected amines,are also tolerated in this method (entries 7-9,Table 2).
| Table 2 Synthesis of substituted dibenzodiazepinones from ethyl 2-halobenzoates and o-phenylenediamine.a |
| Table 2(Continued) Synthesis of substituted dibenzodiazepinones from ethyl 2-halobenzoates and o-phenylenediamine.a |
We have developed an efficient method for the synthesis of dibenzodiazepinones by a one-pot procedure under mild conditions. A variety of functional groups were tolerated,which makes this process very promising for synthesizing a range of substituted dibenzodiazepinones in good yields. Considering the inexpensive catalyst and ligand free conditions,this method could easily be applied for accessing dibenzodiazepine derivatives. Acknowledgment
We are grateful to National Natural Science Foundation of China (No. 81102322) and the Global Alliance for TB Drug Development (TB alliance) for their financial supports.
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.04.049
| [1] | (a) H. Gourdeau, J.B. McAlpine, M. Ranger, et al., Identification, characterization and potent antitumor activity of ECO-4601, a novel peripheral benzodiazepine receptor ligand, Cancer Chemother. Pharmacol. 61(2008) 911-921;(b) J.F. Liegeois, J. Bruhwyler, J. Damas, et al., New pyridobenzodiazepine derivatives as potential antipsychotics: synthesis and neurochemical study, J. Med. Chem. 36(1993) 2107-2114. |
| [2] | C.D. Dotson, L. Zhang, H. Xu, et al., Bitter taste receptors influence glucose homeostasis, PLoS ONE 3(2008) 3974-3983. |
| [3] | (a) Y. Sanchez, C. Wong, R.S. Thoma, et al., Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25, Science 277(1997) 1497-1501;(b) Z. Xiao, Z. Chen, A.H. Gunasekera, et al., Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents, J. Biol. Chem. 278(2003) 21767-21773; |
| [4] | (a) S. Minucci, P.G. Pelicci, Histone deacetylase inhibitors and the promise of epigenetic(and more) treatments for cancer, Nat. Rev. Cancer 6(2006) 38-51;(b) A.J. de Ruijter, A.H. van Gennip, H.N. Caron, et al., Histone deacetylases(HDACs): characterization of the classical HDAC family, Biochem. J. 370(2003) 737-749. |
| [5] | (a) T. Bertomeu, V. Zvereff, A. Ibrahim, et al., TLN-4601 peripheral benzodiazepine receptor(PBR/TSPO) binding properties do not mediate apoptosis but confer tumor-specific accumulation, Biochem. Pharmacol. 80(2010) 1572-1579;(b) R.D. Charan, G. Schlingmann, J. Janso, et al., Diazepinomicin, a new antimicrobial alkaloid from a marine Micromonospora sp., J. Nat. Prod. 67(2004) 1431-1433. |
| [6] | G.A. Kraus, P. Liu, A direct route to the pyrrolo[2,1-c][1,4]benzodiazepine ring system using aryl triflates, Tetrahedron Lett. 42(1995) 7595-7598. |
| [7] | K.C. Majumdar, S. Ganai, CuI/L-proline-catalyzed intramolecular aryl amination: an efficient route for the synthesis of 1,4-benzodiazepinones, Synlett 13(2011) 1881-1887. |
| [8] | (a) E.C. Cortes, P.M. Islas, M.M. Garcia, et al., Synthesis and spectral properties of isomeric [(12-N-methyl) and(10-N-methyl)]-11-(o, and p-substituted-anilino)-5H-dibenzo[b,e][1,4]diazepines, J. Heterocycl. Chem. 33(1996) 1723-1726;(b) R.A. Bunce, J.E. Schammerhorn, Dibenzo-fused seven-membered nitrogen heterocycles by a tandem reduction-lactamization reaction, J. Heterocycl. Chem. 43(2006) 1031-1035. |
| [9] | L. Wang, G.M. Sullivan, L.A. Hexamer, et al., Design, synthesis, and biological activity of 5,10-dihydro-dibenzo[b,e][1,4]diazepin-11-one-based potent and selective Chk-1 inhibitors, J. Med. Chem. 50(2007) 4162-4176. |
| [10] | D. Tsvelikhovsky, S.L. Buchwald, Concise palladium-catalyzed synthesis of dibenzodiazepines and structural analogues, J. Am. Chem. Soc. 133(2011) 14228-14231. |