Red emitting materials can find potential application in some optoelectronic devices such as organic light-emitting diodes [1]. For the emissive materials used in optoelectronic devices,it is desirable that they have the merits including high luminescent quantum yields,good thermal and morphological stabilities,and large Stokes shifts. Thieno-[3,4-b]-pyrazine is one typical red fluorophore that is characterized by high fluorescent quantum yield,good thermal stability,and large Stokes shift of over 100 nm [2]. However,the planar structure of thieno-[3,4-b]- pyrazine often leads to a dramatic decrease in fluorescence intensity in the solid state and poor solubility,owing to the strong π-π stacking interaction [3]. It is a typical strategy to tune the properties of the planar molecules by modifying the molecular conformation. For example,bulky arms are usually incorporated around the emissive core so that the site-isolation effect minimizes the undesired core-core interaction and prevents self-aggregation and concentration-quenching in the solid state [4]. Tetraphenylethylene (TPE) is a typical building block to construct organic functional molecules due to its bulky feature [5]. In the present letter,we report the synthesis of two novel TP derivatives for potential application as red emitting materials. TPE group was introduced into the 2,3,5,7-positions of the thieno-[3,4-b]- pyrazine core in order to protect the core via site-isolation effect and to generate non-planar emissive molecules [6]. The linkage between the emissive core and the peripheral TPE groups is either in a conjugated structure through an ethyne bond (TP-E),or in a non-conjugated mode through an ether structure (TP-O). The structures of the new compounds are shown in Scheme 1.
|
Download:
|
| Scheme 1. Chemical structures of TP-E and TP-O. | |
Chemicals,reagents and solvents from commercial sources are of analytical or spectroscopy grade and used as received without further purification. 1H NMR and 13C NMR spectra were recorded on a Varian INOVA spectrometer (400 MHz for 1H and 100 MHz for 13C). Mass spectra were recorded on a GC-Tof MS (Micromass,UK) mass spectrometer for TOF-MS-EI,a MALDI micro MX (Waters, USA) for MALDI-TOF-MS and HP 1100 LC-MSD (USA) mass spectrometer. The UV-vis absorption and fluorescence spectra measurements were recorded on a Perkin-Elmer Lambda 35 UV- visible spectrophotometer and a Perkin-Elmer LS55 fluorescence spectrometer,respectively. The fluorescence quantum yields were determined against rhodamine B as the standard (Φ = 0.99 in ethanol) [3]. Cyclic voltammetry was performed by using a conventional three-electrode configuration and an electrochemical workstation (BAS 100B,USA) at a scan rate of 50 mV/s. A (0.10 mol/L AgNO3)/Ag electrode and a platinum wire were used as reference and counter electrodes. All measurements were made at room temperature in dichloromethane solutions,with 0.10 mol/L tetra-n-butylammonium hexafluorophosphate (Bu4NPF6) as supporting electrolyte.
The important intermediates 1 [2],2 and 5 [5] were synthesized
and characterized according to the literature methods.
Synthesis of compounds 3 and 4 [2, 7]: To a solution of compound 2 (1 g,2.44 mmol),Pd(PPh3)4 (85 mg,3 mol%),CuI (18.6 mg, 0.1 mmol),and piperidine (20 mL),was added (triisopropylsilyl) acetylene (1.1 mL) under nitrogen. The mixture was stirred under nitrogen at 45 ℃ for 48 h. After removing the solvent and then column chromatography,a white solid (937 mg,yield 75%) of compound 3 was obtained,which was used as reactant directly for the deprotection of the ethyne. A solution of compound 3 (900 mg, 1.77 mmol),tetrahydrofuran (10 mL),and n-BuNF (926 mg, 3.54 mmol) was stirred for 2 h at room temperature. Upon removing the solvent under reduced pressure and then column chromatography on silica gel,a pure product of compound 4 (595 mg,yield 95%) was obtained. EI-MS-TOF (m/z): 356.14 [M]+.
Synthesis of compounds 6 and 7: A solution of 4,4'-dibromodiphenyl ether (400 mg,1.22 mmol),compound 5 (560 mg, 1.22 mmol),Pd(PPh3)4 (43 mg,0.037 mmol) in toluene (10 mL) and methanol (2 mL) and 20% aqueous K2CO3 (4.0 mL) was refluxed under nitrogen for 2 h. Upon removing the solvent,the residue was isolated by column chromatography over silica gel to produce the pure product of compound 6 (374 mg,yield 53%). EIMS- TOF (m/z): 578.1241 [M]+. To the solution of compound 6 (200 mg,0.346 mmol) in tetrahydrofuran (8 mL) at -78 ℃ was added dropwise 2.5 mol/L n-BuLi in hexane (0.28 mL,0.692 mmol) under nitrogen. After the mixture was stirred for 3 h,trimethylborate (0.32 mL) was added dropwise. The liquid nitrogen bath was removed and the mixture was then stirred for another 12 h at room temperature. Upon neutralizing with dilute HCl,the reaction mixture was exacted with dichloromethane. The combined organic layer was dried with anhydrous MgSO4 and evaporated under reduced pressure. The residue was purified by column chromatography to yield compound 7 (110 mg,yield 58.4%),which was put into the next reaction directly without further characterization.
Synthesis of TP-E: A solution of compound 1 (200 mg, 0.264 mmol),4 (566 mg,1.58 mmol),Pd(OAc)2 (22 mg,0.1 mmol), PPh3 (34.3 mg,0.13 mmol) and piperidine (20 mL) was fully deoxygenated and refluxed for 24 h under nitrogen. Upon cooling to room temperature,removing the solvent under reduced pressure,the residue was treated by column chromatography over silica gel to give the pure product of TP-E (256 mg) as a red solid,yield 52%. 1HNMR (400 MHz,CDCl3): δ 8.29 (d,4H,ArH),7.60 (d,4H,ArH),7.53 (d,4H,ArH),7.48 (d,4H,ArH),7.30 (m,8H,ArH), 7.10 (m,36H,ArH),7.04 (m,32H,ArH). 13C NMR (100 MHz,CDCl3): δ 151.90,144.25,144.11,143.52,143.49,143.45,143.42,143.35, 143.32,141.79,141.73,140.29,140.25,139.17,138.55,132.90, 132.06,131.40,131.33,131.31,131.04,131.00,129.86,127.85, 127.77,127.67,127.52,126.72,126.63,124.26,122.77,121.02, 120.81,91.50,91.30,89.84,89.30. MALDI-TOF-MS (m/z): Calcd. for C142H92N2S: 1856.6981; found: 1856.8077 (25%),1857.8209 (75%), 1858.8342 (100%),1859.8182 (82%),1860.8322 (50%).
Synthesis of TP-O: A solution containing compound 1 (100 mg, 0.132 mmol),7 (370.1 mg,0.68 mmol),Pd(PPh3)4 (40 mg, 0.035 mmol),toluene (6 mL),methanol (2 mL) and 20% aqueous K2CO3 (0.8 mL) was refluxed under nitrogen for 12 h. After cooling to room temperature,removing the solvent under reduced pressure,the residue was isolated by column chromatography over silica gel to produce the pure TP-O (192 mg),yield 60%. 1H NMR (400 MHz,CDCl3): δ 8.42 (d,4H,ArH),7.35-7.73 (m,28H, ArH),7.33 (m,8H,ArH),7.06-7.73(m,84H,ArH). 13C NMR (100 MHz,CDCl3): δ 157.22,157.06,156.48,156.41,152.16, 143.75,143.73,142.64,142.62,141.15,140.92,140.52,140.50, 139.65,139.05,138.13,138.09,138.07,136.08,138.03,135.61, 135.35,132.24,131.85,131.53,131.36,131.06,130.50,128.42, 128.24,127.89,127.78,127.71,127.66,127.24,126.50,126.44, 125.96,119.31,119.09,119.07,118.96. MALDI-TOF-MS (m/z): Calcd. for C182H124N2O4S: 2432.948; found: 2432.8608 (65%), 2433.8516 (90%),2434.8420 (100%) 2435.8677 (80%),2436.8247 (55%).
Scheme 2 illustrates the synthetic routes of TP-E and TP-O. They were synthesized by attaching different peripheral tetraphenylethylene groups to the same thieno-[3,4-b]-pyrazine-based core, i.e.,compound 1. The common intermediate 1 that contains the reactive halogen atoms at the surface was synthesized according to the method reported previously by our group [2b]. For the synthesis of the target compound TP-E,the key intermediate 4 was first prepared by incorporation of the terminal ethyne to the one phenyl ring of tetraphenylethylene group. Then TP-E was prepared at a moderate yield of 52% by a reaction between compounds 4 and 1 in piperidine with Pd(OAc)2-PPh3 as catalyst. While for the synthesis of TP-O,the intermediate 6 was first prepared via the Suzuki coupling reaction between the boronic acid 5 and the equivalent mole of 4,4'-dibromodiphenyl ether,then it was converted to the corresponding boronic acid 7. Finally the target compound TP-O was obtained by the Suzuki Coupling reaction between intermediates 7 and 1 at a yield of 60%. Both TP-E and TPO have good solubilities in common organic solvents,so that they were easily purified by column chromatography. Their chemical structures were confirmed by 1H NMR and 13C NMR and highresolution mass spectra.
|
Download:
|
| Scheme 2. Synthetic routes of the important intermediates and target compounds. | |
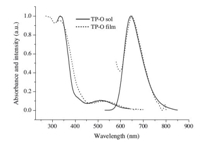
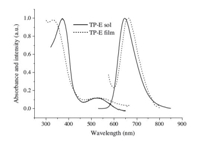
The photophysical spectra of TP-E and TP-O were investigated by measuring the electronic absorption and fluorescence spectra of both their dilute solutions in dichloromethane and their thin films on quartz substrates. Fig. 1 depicts the absorption and fluorescence spectra for TP-O solution and film,and the absorption and fluorescence spectra for TP-E solution and film are shown in Fig. 2. Both the absorption and the fluorescence spectra of these two molecules have almost identical spectral profiles,due to the similarmolecular skeletons for these two molecules. The intense absorption below 400 nm for the compounds could be assigned to π-π* transition of thieno-[3,4-b]-pyrazine core,while the weak absorption band between 500 and 600 nm could be attributed to charge transfer (CT) transitions [2a]. The two absorption peaks are at 372 nm and 530 nm for TP-E solution, and at 334 nm and 513 nm for TP-O solution,respectively. The small red-shift observed in absorption of TP-E in comparison with TP-O is in agreement with the longer conjugation caused by the presence of acetylene bonds in the four peripheral arms within the TP-E molecule. As shown in Figs. 1 and 2,the absorption spectra for the thin films of both TP-O and TP-E did not show significant shift relative to the corresponding solution, indicating the intermolecular interaction that typically exists in the solid state has been successfully suppressed by the bulky arms in these molecules. Upon photoexcitation at 520 nm,both molecules emit strong red fluorescence in dilute dichloromethane solutions with emission peaks at 645 nm. Similar to the absorption spectra,the fluorescence of TP-O film is almost identical to its solution in terms of wavelength. A small red shift of 15 nm was observed in fluorescence of TP-E film relative to its solution. These two compounds exhibit relatively low fluorescence quantum yields (ΦF) of 18.9 and 16.3 in dilute dichloromethane solutions for TP-E and TP-O,respectively. However, the fluorescence quantum yields increase to 31.7 and 23.4 in their toluene solutions are much higher than those in dichloromethane. The increased fluorescence quantum yields in less polar solvent indicate that the intramolecular charge transfer feature exists within these two molecules. The fluorescence lifetimes (τF) of TP-E and TP-O were determined as ca. 3.4 ns and 4.6 ns in dilute dichloromethane solutions at room temperatures.
|
Download:
|
| Fig. 1. Electronic absorption and fluorescence spectra of TP-O in dilute dichloromethane solution (1 × 10-5 mol/L,λexc = 520 nm) and thin film. | |
|
Download:
|
| Fig. 2. Electronic absorption and fluorescence spectra of TP-E in dilute dichloromethane solution (1 × 10-5 mol/L,λexc = 520 nm) and thin film. | |
There is little overlap between the absorption and emission spectra for these two molecules,thus self-absorption can be dramatically eliminated,which is a very important attribute for materials used in optoelectronic devices. Calculated from the positions of the long-wavelength absorption peak and the fluorescence maximum,the Stokes shifts of these two compounds are as large as 115 nm and 132 nm for TP-E and TP-O in solutions, respectively. Such a large Stokes Shift is especially valuable for luminescent materials used in light-emitting devices,since less,or the absence of self-absorption and consequently high light-output can be expected. Based on the saturated red emission,moderate quantum yields,and large Stokes shifts,TP-E and TP-O should be good light-emitting materials for potential application in optoelectronic devices such as light-emitting diodes.
The redox behavior of TP-E and TP-O was investigated by means of cyclic voltammetry measurements. The cyclic voltammograms are provided in Fig. 3. Single electron reduction waves were detected for both TP-E and TP-O,which should be assigned to the reduction of the thieno-[3,4-b]-pyrazine core due to its electrondeficient feature relative to the groups in these molecules [6]. In addition to the reduction waves,at least one reversible oxidation wave was also observed for these two molecules during the anodic sweeping. These processes may be ascribed to the oxidation of the peripheral TPE or other aromatic groups. The highest occupied molecular orbital (HOMO) energies were calculated from the onset potentials of the first oxidation (Eonsetox) using the following equation of HOMO (eV) = -e (Eonsetox + 4.4V) to be 5.36 eV and 5.4 eV for TP-O and TP-E,respectively [6]. The optical band gaps (Eg) were determined by the absorption edge technique using the equation of Eg = 1240/λedge to be ca. 1.94 eV and 1.90 eV for TP-O and TP-E,respectively. Finally,the LUMO energies were calculated by subtracting the gap from the HOMO energy to be 3.42 eV and 3.5 eV for TP-O and TP-E,respectively. Apparently,the relatively deep LUMO levels for these two molecules indicate their strong electron-deficient features,which should originate from the strong electron-withdrawing thieno-[3,4-b]-pyrazine core.
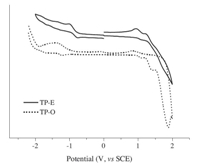
|
Download:
|
| Fig. 3. Cyclic voltammograms of TP-O and TP-E at a scan rate of 50 mV/s in CH2Cl2. | |
Two novel thieno-[3,4-b]-pyrazine based molecules with four large tetraphenylethylene arms were synthesized and characterized. The photophysical study revealed that these compounds emit saturated red fluorescence with moderate quantum yields and large Stokes shifts of over 100 nm. It is expected these compounds can find potential application as non-doped luminescent materials in optoelectronic devices such as light-emittng diodes.
We thank the National Natural Science Foundation of China (Nos. 21274016 and 21072026) and Fundamental Research Funds for the Central Universities (No. DUT13LK06) for financial support to this work.
| [1] | C.D. Muller, A. Falcou, N. Reckefuss, et al., Multi-colour organic light-emitting displays by solution processing, Nature 421(2003) 829-833. |
| [2] | K.R.J. Thomas, J.T. Lin, Y.T. Tao, C.H. Chuen, Star-shaped thieno-[3 4-b]-pyrazines: a new class of red-emitting electroluminescent materials, Adv. Mater. 14(2002) 822-826. |
| [3] | Q. Li, J. Li, H. Ren, et al., Solution-processible thieno-[3 4-b]-pyrazine derivatives with large stokes shifts for non-doped red light-emitting diodes, Macromol. Rapid Commun. 32(2011) 736-743. |
| [4] | (a) C. Liu, Y.H. Li, Y.Y. Zhang, et al., Solution-processed, undoped, deep-blue organic light-emitting diodes based on starburst oligofluorenes with a planar triphenylamine core, Chem. Eur. J. 18(2012) 6928-6934; |
| [5] | (a) W.Z. Yuan, P. Lu, S. Chen, et al., Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: development of highly efficient light emitters in the solid state, Adv. Mater. 22(2010) 1-5;(b) Z. Zhao, S. Chen, J.W.Y. Lam, et al., Creation of highly efficient solid emitter by decorating pyrene core with AIE-active tetraphenylethene peripheries, Chem. Commun. 46(2010) 2221-2223. |
| [6] | J. Li, Y. Duan, Q. Li, Novel thieno-[3 4-b]-pyrazine derivatives for non-doped red organic light-emitting diodes, Dyes Pigments 96(2013) 391-396. |
| [7] | Q. Li, J. Li, R. Yang, et al., Novel red-emitting thieno-[3 4-b]-pyrazine derivatives suitable for vacuum evaporation and solution method to fabricate non-doped OLEDs, Dyes Pigments 92(2011) 674-680. |




