b Wenzhou Vocational and Technical College, Wenzhou 325035, China;
c College of Pharmaceutical and Chemical Engineering, Taizhou University, Linhai 317000, China
Pyrrole derivatives,as an essential class of heterocycles,have become increasingly important in the past few years because they have been associated with a wide range of pharmacological and biological activities [1, 2, 3]. A number of synthetic methods to prepare these compounds have been developed in recent years [4, 5, 6, 7, 8, 9, 10, 11]. One of the most classical and common approaches for the synthesis of N-substituted pyrrole derivatives involves the Paal- Knorr condensation reaction of γ-diketones with amines in the presence of various promoting agents including amberlite IR 120 acidic resin [12],montmorillonite [13],p-toluenesulfonic acid [14], silica sulfuric acid [15],PEG-bounded sulfonic acid [16],xanthan sulfuric acid [17] silica-supported SbCl3 [18],polystyrene-supported GaCl3 [19] and different metal complexes [20, 21, 22, 23, 24]. Additionally,the above cyclo-condensation process could proceed in ionic liquid [25],microwave irradiation [13, 26],ultrasonic irradiation [27] and infrared irradiation [28]. In spite of their potential utility,however,the use of high temperatures,an excess of hazardous organic solvents,and expensive catalysts that are harmful to the environment,unsatisfactory yields,and cumbersome product isolation procedures limit the use of these methods. Therefore,the development of efficient,practical and facile methods for the synthesis of N-substituted pyrrole derivatives is still desirable.
β-Cyclodextrin,a commercially available reagent,has been used for various organic functional group transformations as catalyst [29, 30]. To the best of our knowledge,until now,β-cyclodextrin- mediated Paal-Knorr reaction has never been studied or reported.
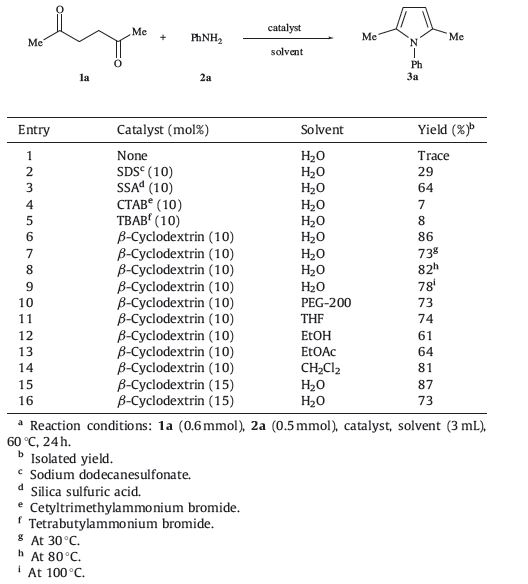
As part of the continuing efforts in our laboratory towards the development of new methods for the synthesis of nitrogencontaining heterocycles [11, 20, 21, 22, 31, 32, 33, 34],we herein report a new and efficient method for the synthesis of N-substituted pyrrole derivatives by the Paal-Knorr reaction in the presence of β-cyclodextrin in aqueous media (Scheme 1).
|
Download:
|
| Scheme 1. β-Cyclodextrin-mediated Paal-Knorr reaction in water. | |
Chemicals were purchased and used without further purification. 1H NMR and 13C NMR spectra were measured on a 500 MHz Bruker spectrometer,using CDCl3 as the solvent with tetramethylsilane (TMS) as the internal standard at room temperature. Chemical shifts are reported in δ relative to TMS,and coupling constants J are in Hz. Column chromatography was performed using EM Silica gel 60 (300-400 mesh). All compounds are known, and their characterization data and spectra (1H NMR and 13C NMR) can be found in the Supporting information.
General synthetic procedure for the preparation of n-substituted pyrroles 3: To a solution of γ-diketone 1 (0.6 mmol) and amine 2 (0.5 mmol) in H2O (3 mL),β-cyclodextrin (10 mol%) was added. The mixture was stirred for 24 h at 60 ℃. After the completion of the reaction,as monitored by TLC and GC-MS analysis,the reaction mixture was cooled to room temperature,diluted in ethyl acetate, and washed with brine. The aqueous phase was re-extracted with ethyl acetate. The combined organic extracts were dried over Na2SO4 and concentrated in vacuum,and the resulting residue was purified by silica gel column chromatography (petroleum ether/ ethyl acetate) to afford the desired product 3.
Initially,the reaction between hexane-2,5-dione (1a) and aniline (2a) was chosen as a model reaction to screen for the optimal reaction conditions (Table 1). Our investigation began with the model reaction run in water in the absence of catalysts in which the target product,2,5-dimethyl-1-p-phenyl-1H-pyrrole (3a),was detected in trace amounts (entry 1). The yield of 3a was enhanced to some extent when the reaction was conducted in the presence of various catalysts,such as SDS,SSA,CTAB,TBAB,and bcyclodextrin in aqueous media (entries 2-6). Among these catalysts,the reaction which afforded 3a in the highest yield uses β-cyclodextrin as the catalyst at 60 ℃ (86% yield,entry 6). The yield was decreased to some extent when the reaction was carried out at lowered or elevated temperatures (entries 7-9). Similarly, the effect of solvents was also tested. Among all the solvents screened (e.g.,H2O,THF,PEG-200,EtOH,EtOAc and CH2Cl2),H2O was determined to be the most effective medium for the generation of the desired products (entries 6,10-16). Moreover, we also studied the influence of the amount of catalyst on the reaction yields. A 10 mol% of β-cyclodextrin was sufficient and excess amounts of catalyst did not increase the yield remarkably (entries 6,15-16).
| Table 1 Screening for optimal reaction conditions.a |
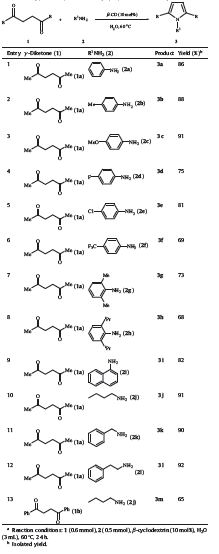
With the optimal conditions in hand,the Paal-Knorr reaction of γ-diketones (1) with various substituted aniline (2) derivatives and several amines was examined to explore the scope of the present protocol and the results are summarized in Table 2.
| Table 2 Paal-Knorr pyrroles synthesis using β-cyclodextrin in aqueous media.a |
As shown in Table 2,a series of aromatic amines bearing either electron-donating or electron-withdrawing groups were investigated. In general,electron-rich aromatic amines afforded a higher yield than the electron-deficient aromatic amines. For example,it was observed that electron-donating groups on the phenyl ring of aromatic amines such as 2b and 2c favoured the formation of the corresponding products 3b and 3c in good yields (entries 2 and 3). In contrast,electron-withdrawing groups associated with aromatic amines such as 2d-2f slightly decreased the reactivity of the substrate (entries 4-6). Gratifyingly,the sterically hindered amines such as 2,6-dimethylaniline (2g) and 2,6-diisopropylaniline (2h) smoothly reacted with 1a under the standard conditions to give the corresponding products 3g and 3h in 73% and 68% yields,respectively (entries 7 and 8). The transformation of 1- aminonaphthalene (2l) with 1a also proceeded successfully to provide the desired product 3l in good yield (entry 9). On the other hand,aliphatic amines 2j-2l were treated with 1a under standard conditions to afford the corresponding products in excellent yields (entries 10-12). The corresponding product 3m was obtained in 65% yield when 1,4-diphenylbutane-1,4-dione (1b) was used as substrate (entry 13).
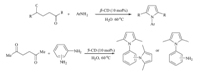
Next,the Paal-Knorr reactions of diamines with 1a were investigated (Scheme 2). In this reaction,two equivalents of 1a were required in order to have a complete conversion of diamines. When aromatic diamines,such as para-phenyleneamine or metaphenyleneamine were examined,the corresponding bis-pyrrole products 3n and 3o were obtained in 74% and 72% yields, respectively. However,when the more sterically hindered aromatic diamine,such as para-phenylenediamine,was examined, the reaction gave only the mono-pyrrole product 3p in 63% yield in the presence of two equivalents,and no improvement even with three equivalents 1a.
|
Download:
|
| Scheme 2. Reaction of hexane-2,5-dione with phenylenediamines. Reaction conditions: 1a (2.4 mmol),2 (1 mmol),β-CD (20 mol%) and H2O (3 mL),24 h,60 ℃. | |
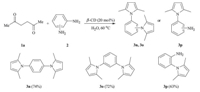
Finally,we investigated the recycling of the β-cyclodextrin. Subsequent Paal-Knorr reactions,for example,of hexane-2,5- dione (1a) and aniline (2a) shown in Scheme 3 confirmed (demonstrated) β-cyclodextrin could be was reclaimed for four recycles without a significant loss in catalytic activity.

|
Download:
|
| Scheme 3. Recyclability of β-cyclodextrin. Run 1: 84%; Run 2: 81%; Run 3: 79%; Run 3:79%; Run4: 78%. | |
A tentative mechanism for the formation of N-substituted pyrroles is proposed in Scheme 4. The role of the β-cyclodextrin facilitates the formation of N-substituted pyrroles via inclusion of γ-diketones and stabilized by the primary and secondary-OH groups of β-cyclodextrin,which further reacts with amines and subsequent cyclization followed by elimination leads to the N-substituted pyrroles 3.
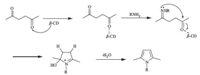
|
Download:
|
| Scheme 4. Possible mechanism for the formation of N-substituted pyrroles. | |
In summary,we have developed a new catalytic protocol to synthesize N-substituted pyrroles by Paal-Knorr reaction of γ-diketones with amines in the presence of β-cyclodextrin in aqueous media. The application of the developed approach is successfully explored towards the Paal-Knorr reaction of hexane- 2,5-dione with diamines,leading to the corresponding bis-pyrroles or mono-pyrrole in impressive yields. Further efforts to expand the scope of the chemistry are currently underway in our laboratory.
Wethank the National Natural Science Foundation of China (No. 21172175) and Natural Science Foundation of Zhejiang Province (Nos. LY12B02011 and Y4110491) for financial support.
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013. 05.012.
| [1] | R. Rango, G.R. Marshal, R. Di Santo, et al., Antimycobacterial pyrroles: synthesis, anti-Mycobacterium tuberculosis activity and QSAR studies, Bioorg. Med. Chem. 8(2008) 1423-1432. |
| [2] | B. Pacorel, S.C. Leung, A.V. Stachulski, et al., Modular synthesis and in vitro and in vivo antimalarial assessment of C-10 pyrrole mannich base derivatives of artemisinin, J. Med. Chem. 53(2010) 633-640. |
| [3] | T. Watanabe, Y. Umezawa, Y. Takahashi, Y. Akamatsu, Novel pyrrole-and 1,2,3-triazole-based 2,3-oxidosqualene cyclase inhibitors, Bioorg. Med. Chem. Lett. 20(2010) 5807-5810. |
| [4] | L. Kürti, B. CzakóZ, Strategic Applications of Named Reactions in Organic Synthesis, Elsevier, Amsterdam, 2005, pp. 328-329. |
| [5] | V. Estévez, M. Villacampa, J.C. Menéndez, Multicomponent reactions for the synthesis of pyrroles, Chem. Soc. Rev. 39(2010) 4402-4421, and references cited therein. |
| [6] | S. Michlik, R. Kempe, A sustainable catalytic pyrrole synthesis, Nat. Chem. 5(2013) 140-144. |
| [7] | M. Zhang, H. Neumann, M. Beller, Selective ruthenium-catalyzed three-component synthesis of pyrroles, Angew. Chem. Int. Ed. 52(2013) 597-601. |
| [8] | B. Li, N.C. Wang, Y.J. Liang, S.S. Xu, B.Q. Wang, Ruthenium-catalyzed pyrrole synthesis via oxidative annulation of enamides and alkynes, Org. Lett. 15(2013) 136-139. |
| [9] | W.Z. Geng, W.X. Zhang, W. Hao, Z.F. Xi, Cyclopentadiene-phosphine/palladiumcatalyzed cleavage of C-N bonds in secondary amines: synthesis of pyrrole and indole derivatives from secondary amines and alkenyl or aryl dibromides, J. Am. Chem. Soc. 134(2012) 20230-20233. |
| [10] | D. Dhara, K.S. Gayen, S. Khamarui, et al., CeCl>3>·7H2O catalyzed C-C and C-N bondforming cascade cyclization with subsequent side-chain functionalization and rearrangement: a domino approach to pentasubstituted pyrrole analogues, J. Org. Chem. 77(2012) 10441-10449. |
| [11] | H.J. Deng, Y.J. Fang, G.W. Chen, et al., Copper-catalyzed Clauson-Kass pyrroles synthesis in aqueous media, Appl. Organomet. Chem. 26(2012) 164-167. |
| [12] | H.R. Darabi, M.R. Poorheravi, K. Aghapoor, et al., Silica-supported antimony(Ⅲ) chloride as a mild and reusable catalyst for the Paal-Knorr pyrrole synthesis, Environ. Chem. Lett. 10(2012) 5-12. |
| [13] | M. Abid, A. Spaeth, B. Török, Solvent-free solid acid-catalyzed electrophilic annelations: a new green approach for the synthesis of substituted five-membered N-heterocycles, Adv. Synth. Catal. 348(2006) 2191-2196. |
| [14] | S. Raghavan, K. Anuradha, Solid-phase synthesis of heterocycles from 1,4-diketone synthons, Synlett 2003(2003) 711-713. |
| [15] | H. Veisi, Silica sulfuric acid(SSA) as a solid acid heterogeneous catalyst for onepot synthesis of substituted pyrroles under solvent-free conditions at room temperature, Tetrahedron Lett. 51(2010) 2109-2114. |
| [16] | A.A. Jafari, S. Amini, F. Tamaddon, A green, chemoselective, and efficient protocol for Paal-Knorr pyrrole and bispyrrole synthesis using biodegradable polymeric catalyst PEG-SO3H in water, J. Appl. Polym. Sci. 125(2012) 1339-1345. |
| [17] | A. Rahmatpour, Xanthan sulfuric acid as an efficient, green, biodegradable, and recyclable solid acid catalyst for one-pot synthesis of N-substituted pyrroles under solvent-free conditions at roomtemperature, Monatsh Chem. 143(2012) 491-495. |
| [18] | A. Devi, M. Shallu, L. Sharma, J. Singh, Paal-Knorr pyrrole synthesis using recyclable amberlite IR 120 acidic resin: a green approach, Synth. Commun. 42(2012) 1480-1488. |
| [19] | A. Rahmatpour, Polystyrene-supported GaCl3 as a highly efficient and recyclable heterogeneous Lewis acid catalyst for one-pot synthesis of N-substituted pyrroles, J. Organomet. Chem. 712(2012) 15-19, and references cited therein. |
| [20] | J.X. Chen, H.Y. Wu, Z.G. Zheng, et al., An approach to the Paal-Knorr pyrroles synthesis catalyzed by Sc(OTf)3 under solvent-free conditions, Tetrahedron Lett. 47(2006) 5383. |
| [21] | J.X. Chen, M.C. Liu, X.L. Yang, J.C. Ding, H.Y. Wu, Indium(Ⅲ)-catalyzed synthesis of N-substituted pyrroles under solvent-free conditions, J. Braz. Chem. Soc. 19(2008) 877-883. |
| [22] | J.X. Chen, X.L. Yang, M.C. Liu, et al., Approach to synthesis of β-enamino ketones and pyrroles catalyzed by gallium(Ⅲ) triflate under solvent-free conditions, Synth. Commun. 39(2009) 4180-4198. |
| [23] | B. Temelli, C. Unaleroglu, A novel method for the synthesis of dipyrromethanes by metal triflate catalysis, Tetrahedron 62(2006) 10130-10135. |
| [24] | B.K. Banik, I. Banik, M. Renteria, S.K. Dasgupta, A straightforward highly efficient Paal-Knorr synthesis of pyrroles, Tetrahedron Lett. 46(2005) 2643-2645. |
| [25] | B. Wang, Y.L. Gu, C. Luo, et al., Pyrrole synthesis in ionic liquids by Paal-Knorr condensation under mild conditions, Tetrahedron Lett. 45(2004) 3417-3419. |
| [26] | G.E. Veitch, K.L. Bridgwood, K. Rands-Trevor, S.V. Ley, Magnesium nitride as a convenient source of ammonia: preparation of pyrroles, Synlett 2008(2008) 2597-2600. |
| [27] | Z.H. Zhang, J.J. Li, T.S. Li, Ultrasound-assisted synthesis of pyrroles catalyzed by zirconium chloride under solvent-free conditions, Ultrason. Sonochem. 15(2008) 673-676. |
| [28] | C. Zhang, J. Wang, J.H. Li, Infrared heat aided solid state synthesis of pyrroles from 1,4-diketones and ammonium acetate, J. Heterocycl. Chem. 49(2012) 204-207. |
| [29] | K. Ramesh, K. Karnakar, G. Satish, K.H.V. Reddy, Y.V.D. Nageswar, Tandem supramolecular synthesis of substituted 2-aryl-2,3-dihydroquinazolin-4(1H)-ones in the presence of β-cyclodextrin in water, Tetrahedron Lett. 53(2012) 6095-6099. |
| [30] | Y.L. Hu, H. Jiang, M. Lu, Efficient and convenient C-3 functionalization of indoles through Ce(OAc)3/TBHP-mediated oxidative C-Hbond activation in the presence of β-cyclodextrin, Green Chem. 13(2011) 3079-3087, and references cited therein. |
| [31] | J.X. Chen, W.K. Su, H.Y. Wu, M.C. Liu, C. Jin, Eco-friendly synthesis of 2,3-dihydroquinazolin-4(1H)-ones in ionic liquids or ionic liquid-water without additional catalyst, Green Chem. 9(2007) 972-975. |
| [32] | J. Chen, D. Wu, F. He, et al., Gallium(Ⅲ) triflate-catalyzed one-pot selective synthesis of 2,3-dihydroquinazolin-4(1H)-ones and quinazolin-4(3H)-ones, Tetrahedron Lett. 49(2008) 3814-3818. |
| [33] | D. Huang, J. Chen, W. Dan, et al., A metal-free sulfenylation and bromosulfenylation of indoles: controllable synthesis of 3-arylthioindoles and 2-bromo-3-arylthioindoles, Adv. Synth. Catal. 354(2012) 2123-2128. |
| [34] | J.X. Chen, H.Y. Wu, W.K. Su, A facile synthesis of 2,3-dihydro-2-aryl-4(1H)-quinazolinones catalyzed by scandium(Ⅲ) triflate, Chin. Chem. Lett. 18(2007) 536-538. |