b Institute of Chemical Materials, CAEP, Mianyang 621900, China
Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro- 1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX),as polynitro derivatives of nitrogen-containing heterocycles,are common explosives and used extensively for civil as well as military applications [1, 2, 3, 4]. To discover and develop the most powerful explosives,much effort has been devoted to introducing nitro groups into aromatic nitrogen-containing heterocycles such as azoles,azines and furazans in the past decade [5]. However,some of these polynitro compounds exhibit poor explosive performance and stability. Therefore,the foremost objective to the design of new explosives remains finding molecules having both good energy capability and optimal safety (reduced vulnerability,shock and impact insensitivity), and this research seems to be never ending [6, 7, 8, 9, 10]. Theoretical study shows that tetranitro-4,4'-bi-1,2,4-triazole (TNBT) might be an ideal energetic material with high density (1.99 g/cm3) and detonation velocity (9.50 km/s) [11],but it is still in the stage of synthetic exploration.
Polynitro aromatic nitrogen-containing heterocycles are traditionally synthesized by nitration with nitrating agents or oxidation of amino groups [12, 13, 14]. An effective way to obtain polynitro imidazoles is the iodination of imidazoles followed by nitrolysis [15, 16, 17]. Iodinated molecules have established themselves as versatile intermediates in synthetic organic chemistry owing to the ability of iodo group in carbon-carbon, carbon-nitrogen,carbon-oxygen bond formation [18]. So tetraiodo- 4,4'-bi-1,2,4-triazole (1) as a key intermediate can not only lead to TNBT and its analogs through nitrolysis,but also might produce many valuable derivatives as pharmaceuticals or pesticides if further studied. Herein,we report the synthesis of this key intermediate together with its interesting crystal structure and thermal stability. The research on the nitration and other derivatization reactions is under way and will be reported later.
The elemental analysis and melting point were determined using an Elementar Vario EL cube and an Optimelt OPM100, respectively. The IR spectra were measured on a Nicolet Nexus 870 FTIR spectrophotometer (KBr pellet). HRMS spectra were recorded at a microTOF-Q II mass spectrometer with ESI as the ionization method. Single-crystal X-ray diffraction data of 1 (CCDC 900710) were collected on a Bruker Smart APEX II CCD and the crystallographic data are listed in Table 1. Thermal analysis was carried out on a Simultaneous TGA/DSC (SDT) SDT-Q600.
An iodine monochloride (3.89 g,0.024 mol) aqueous solution (10 mL) was added dropwise to a stirred solution of 2 (0.68 g, 0.005 mol) in water (20 mL) at r.t.,and then the resulting mixture was heated at 60 ℃ and stirred overnight. The mixture was neutralized with a diluted basic solution. The white precipitates were filtered,washed with water (2× 3 mL) and dried under vacuum. Recrystallization from methanol afforded colorless crystals (as shown in Scheme 1). Yield: 2.7 g,4.2 mmol,85%. Mp 275 ℃ (dec.). Anal. calcd. for C4N6I4: C 7.51,N 13.14; found: C 7.42, N 13.24. Selected IR (KBr,cm-1): 1573vs,1500vs,1390s,1315s, 846vs,651.7m. HRMS (ESI) (m/z): Calcd. for C4N6I4Na: 662.6255 [M+Na+],found: 662.6266. Compounds 2 and 3 were obtained using literature procedures [19].
| Table 1 Crystal data and structure refinement for tetraiodo-4,4'-bi-1,2,4-triazole. |

|
Download:
|
| Scheme 1. Synthetic route of tetraiodo-4,4'-bi-1,2,4-triazole (1). | |
A colorless crystal of the title compound was mounted on a glass fiber in a random orientation. The data were collected by a Bruker Smart APEX II CCD diffractometer equipped with a graphite-monochromatic Mo Kα radiation (λ = 0.71075 Å) by using an w scan mode in the range of 2.448° ≤θ ≤30.83° at 293(±2) K. The structure was solved by direct methods with SHELXS-97 program [20] and refined using SHELXL-97 program [20].

|
Download:
|
| Fig. 1. Molecular structure and packing diagram in a unit cell of title compound. | |
The IR,HRMS spectra and elemental analysis data are in good agreement with the assigned structure of compound 1. X-ray structural data show that the title compound is composed of two triazole rings with two iodine atoms on each (Fig. 1). The torsional angles of C(1)- (1)-N(4)-C(3),C(2)-N(1)-N(4)-C(3) are -92.7°(6) and -84.5°(6),respectively (Table 2),and the data indicate that the two triazole rings are perpendicular to each other. This interesting conformation is owing to the bulky iodine atoms with great steric hindrance that render planar conformations unstable. In addition, the 3D structure of the title compound is constructed via aromatic Π•••Π packing interactions,which is beneficial to the solid state stability.
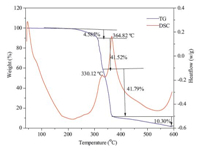
The thermal stability of compound 1 was investigated by thermogravimetric analysis (TGA) and differential scanning calorimeter (DSC) from room temperature to 600 ℃,at a heating rate of 10 ℃/min in a nitrogen atmosphere. The measurements were taken using 1660.00 mg sample. Weight-loss/heat flow/ temperature curves were recorded (as shown in Fig. 2).
In the DSC curve,there are two distinct exothermic peaks. Both are intensely exothermic with the peak temperature at 330.12 ℃ and 364.82 ℃,respectively,which indicates that they are both rapid decomposition processes. Corresponding to these two processes,there are two strongly accelerated and separated weight losses in TGA curve. One occurs from 275.40 ℃ to 338.35 ℃ with a weight loss of 41.52% of the initial weight and the other weight loss of 41.79% occurs from 338.35 ℃ to 364.82 ℃. The weight losses indicate that four iodine atoms in a tetraiodo- 4,40-bi-1,2,4-triazole molecule have been removed from the molecular as iodine at 275.40 ℃ and 338.35 ℃,respectively.
|
Download:
|
| Fig. 2. DSC-TGA curve of tetraiodo-4,4'-bi-1,2,4-triazole. | |
| Table 2 Selected bond lengths (Å ),bond angles (°) and torsion angles (°) for the title compound. |
In summary,teteriodo-4,4'-bi-1,2,4-triazole (1) as a key intermediate for the synthesis of energetic material TNBT was prepared and structurally characterized and the crystals of compound 1 were obtained from methanol. The molecular structure is featured with two perpendicular triazole rings and aromatic Π•••Π packing interactions between molecules. The title compound is thermally stable at room temperature and four iodine atoms in a molecule are removed as iodine at 275.40 ℃ and 338.35 ℃,respectively. Due to the reactivity of the iodo groups, compound 1 might be a useful intermediate in energetic materials and some other fields. It is worthy to research the nitrolysis and other derivatization reactions of teteriodo-4,4'-bi-1,2,4-triazole and the related work will be further reported
This work was supported by the National Key Projects (No. 00402040103) and Youth Innovation Research Team of Sichuan for Carbon Nanomaterials (No. 2011JTD0017).
| [1] | A.K. Sikder, N. Sikder, A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications, J. Hazard. Mater. 112(2004) 1-15. |
| [2] | J.P. Agrawal, Recent trends in high-energy materials, Prog. Energy Combust. Sci. 24(1998) 1-30. |
| [3] | F.P. Philip, S.L. Gregory, R.M. Alexander, R.D. Schmidt, A review of energetic materials synthesis, Thermochim. Acta 318(2002) 187-204. |
| [4] | J.P. Agrawal, R.D. Hodgson, Organic Chemistry of Explosives, Wiley-VCH, Wiltshire, 2007. |
| [5] | (a) S. Nimesh, A.G. Rajendran, Dinitrophenyl triazoles-"a class of energetic azoles": synthesis, characterisation, and performance evaluation studies, Propellants Explos. Pyrotech. 37(2012) 267-274;(b) M.H. Keshavarz, K. Esmailpoor, Theoretical prediction of physicochemical properties, performances and sensitivities of some new derivatives of dinitro triazolyl triazine, Propellants Explos. Pyrotech. 35(2010) 482-486;(c) V.D. Ghule, R. Sarangapani, P.M. Jadhav, S.P. Tewari, Quantum-chemical investigation of substituted s-tetrazine derivatives as energetic materials, Bull. Korean Chem. Soc. 33(2012) 564-570;(d) J.S. Li, Y.G. Huang, H.S. Dong, Theoretical calculation and molecular design for high explosives: theoretical study on polynitropyrazines and their N-oxides, Propellants Explos. Pyrotech. 29(2004) 231-235;(e) R.H. Wang, Y. Guo, Z. Zeng, B. Twamley, J.M. Shreeve, Furazan-functionalized tetrazolate-based salts: a new family of insensitive energetic materials, Chem. Eur. J. 15(2009) 2625-2634;(f) J.R. Li, Research development of furazan energetic materials, Chin. J. Explos. Propel. 3(1998) 56-60. |
| [6] | Y. Zhou, X.P. Long, X. Wang, Y.J. Shu, A.M. Tian, Review on high-nitrogen energetic materials, Chin. J. Energ. Mater. 14(2006) 315-320. |
| [7] | H.X. Gao, C.F. Ye, O.D. Gupta, et al., 2,4,5-Trinitroimidazole-based energetic salts, Chem. Eur. J. 31(2007) 3853-3860. |
| [8] | H. Xue, Y. Gao, B. Twamley, J.M. Shreeve, New energetic salts based on nitrogencontaining heterocycles, Chem. Mater. 17(2005) 191-198. |
| [9] | H. Xue, B. Twamley, J.M. Shreeve, Energetic quaternary salts containing bi(1,2,4-triazoles), Inorg. Chem. 44(2005) 7009-7013. |
| [10] | M.B. Talawar, R. Sivabalan, N. Senthilkumar, G. Prabhu, S.N. Asthana, Synthesis, characterization and thermal studies on furazan-and tetrazine-based high energy materials, J. Hazard. Mater. 113(2004) 11-25. |
| [11] | M.D. Coburn, B.W. Harris, K.Y. Lee, M.M. Stinecipher, H.H. Hayden, Explosives synthesis at Los Alamos, Ind. Eng. Chem. Prod. Res. Dev. 25(1986) 68-72. |
| [12] | S.Q. Yang, S.L. Xu, Y.P. Lei, Development on nitrogen heterocyclic energetic compounds, Chin. J. Energ. Mater. 14(2006) 476-484. |
| [13] | S.S. Novikov, L.I. Khmel'nitskii, O.V. Lebedev, V.V. Sevost'yanaova, L.V. Epishina, The nitration of iodoimidazoles with various nitrating agents, Chem. Heterocycl. Compd. 6(1970) 503-507. |
| [14] | D.M. Badgujar, M.B. Talawar, S.N. Asthana, P.P. Mahulikar, Advances in science and technology of modern energetic materials: an overview, J. Hazard. Mater. 151(2008) 289-305. |
| [15] | H.S. Jadhav, M.B. Talawar, R. Sivabalan, et al., Synthesis, characterization and thermolysis studies on new derivatives of 2,4,5-trinitroimidazoles: potential insensitive high-energy materials, J. Hazard. Mater. 143(2007) 192-197. |
| [16] | A.R. Katritzky, D.J. Cundy, J. Chen, Polyiodoimidazoles and their nitration products, J. Energ. Mater. 11(1993) 345-352. |
| [17] | S.S. Novikov, L.I. Khmel'nitskii, O.V. Lebedev, L.V. Epishina, V.V. Sevost'yanaova, The nitration of iodoimidazoles, Chem. Heterocycl. Compd. 6(1970) 664-668. |
| [18] | M.J. Mphahlele, L.G. Lesenyeho, H.R. Makelane, Synthesis of 1H-pyrrolo[3,2-c]quinoline derivatives via palladium-catalyzed heteroannulation of 2-aryl-3-iodo-4-(phenylamino)quinolines and 4-(N,N-allylphenylamino)-2-aryl-3-iodoquinolines, Tetrahedron 66(2010) 6040-6046. |
| [19] | A.D. Naik, M.B. Jacqueline, Y. Garcia, A simplified approach to N-and N,N'-linked 1,2,4-triazoles by transamination, Synthesis 39(2008) 149-154. |
| [20] | (a) G.M. Sheldrick, SHELXS-97, Program for X-ray Crystal Structure Solution, Göttingen University, Germany, 1997;(b) G.M. Sheldrick, SHELXS-97, Program for X-ray Crystal Structure Refinement, Göttingen University, Germany, 1997. |






