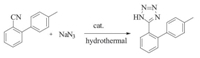
5-(4'-Methylbiphenyl-2-yl)-1H-tetrazole (MBPTZ) is a very important intermediate that has widely been utilized in the synthesis of sartan [1]. Sartan belongs to a class of compounds known as angiotensin II (AT-II) receptor antagonists [2]. This class combines effective anti-hypertensive activity with an excellent profile of safety and tolerability. The therapeutic standard was significantly improved by the introduction of sartan. Because of its wide applications in explosives,organic syntheses,coordination chemistry and pharmacy,many synthetic methods of tetrazole are reported in the literature [3, 4, 5, 6, 7, 8]. It is usually produced by [2+3] cycloaddition of azides with nitriles. A typical procedure for the preparation of tetrazole was reported in 2003 by Sharpless and coworkers [9, 10],using NaN3 and stoichiometric amounts of Zn(II) salts in water from the corresponding nitriles. This procedure is innovative and safe,but complex by-products of Zn(II) on precipitation makes the yield low and difficult to utilize in mass production. With another procedure using Me3SnN3 or Me3SiN3 [11, 12] instead of NaN3,the yield is improved,but toxic heavy metal salt ions were introduced into the reaction system,and extended the reaction route. Recently amberlyst-15,ion liquid,or microwaves were used in tetrazole synthesis [13, 14],but it raised the cost of its production,and can hardly be used in mass production. Therefore,the development of new environmentally friendly procedures for tetrazole synthesis is a very important subject in modern organic synthesis.
Our research group devoted particular attention to the development of environmentally friendly and efficient protocols of tetrazole synthesis [15]. Hydro (solvo) thermal reactions [16], which typically carried out under sealed conditions and in the temperature range 120-200 ℃ and under autogenous pressure (generally 10-30 atm),are known to facilitate the self-assembly of the product from soluble precursors. According to the mechanism proposed by Sharpless and coworkers,the [2+3] cycloaddition reaction of an azide anion and a nitrile group might easily take place in solvents that have good solubility for both. Propane-1,2- diol (DPOL),a safer solvent widely used in many pharmaceutical processes,features a low vapor pressure and good solubility of NaN3 and 4'-methylbiphenyl-2-carbonitrile under hydrothermal conditions. Herein,we report the hydrothermal synthesis of MBPTZ in the solvent of mixed DPOL and water,with ammonium chloride and ammonium fluoride as catalyst.
Commercially available reagents were used as received without further purification. Infrared spectra were taken on a Bruker Vector 22 spectrophotometer as KBr pellets in the 4000-400 cm-1 region. The 1H NMR spectrum was obtained with a Bruker Am-300 spectrometer operating at 300 MHz using DMSO as solvent at room temperature. High performance liquid chromatography (HPLC) was taken on WuFeng LC-100 with mixed acetonitrile and water as mobile phase. Electrospray ionization mass spectrometry (ESI-MS) was obtained with Thermofisher Finnigan LCQ Advantage Max.
|
Download:
|
| Scheme 1. Synthesis route of the compound MBPTZ. | |
As shown in Scheme 1,1 mol of NaN3 dissolved in 150 mL of H2O,together with 1.2 mol of 4'-methylbiphenyl-2-carbonitrile, 1.5 mol of ammonium chloride,0.2 mol of ammonium fluoride and 500 mL of DPOL were sealed in a 1 L pressure vessel,after stirring and heating for 48 h,the pressure gradually increased to 3 atm.
After the reaction,the liquid is cooled to 50 ℃,to decompression the vessel,the pH of released gas is about 8,released gas was absorbed with diluted hydrochloric acid or water. The reaction mixture was light yellow,and needle-like raw material crystals would be precipitated if cooling continued. The pH of reaction solution is about 8,500 mL H2O were added,washed twice with 200 mL toluene or ethyl acetate.
Concentrated hydrochloric acid was trickled into the separated water layer with strong stirring,and a large amount of white solid product was precipitated,HCl addition was continued until no further precipitation,and the pH was about 7. The precipitate was filtered,washed with H2O,and dried at 105 ℃ to give the pure product. Its HPLC and ESI-MS is shown in Fig. S1 and S2 in Supporting information file.
Only 4'-methylbiphenyl-2-carbonitrile was extracted into the organic layer,it can be separated by concentration.
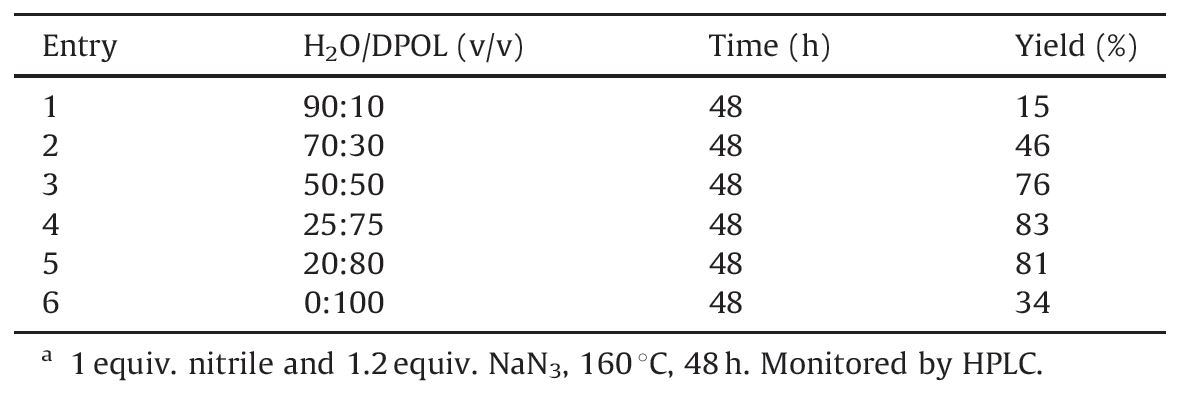
Initially,the ratio of DPOL and water had to be optimized. Water is a good solvent of NaN3 and ammonium salts,but inhibits the dissolution of 4'-methylbiphenyl-2-carbonitrile,while DPOL is the contrary. The best ratio is 10 equiv. of DPOL with 3 equiv. of water according to Table 1. Higher or lower ratios lead to the decrease of the yield due to low solubility of reactant.
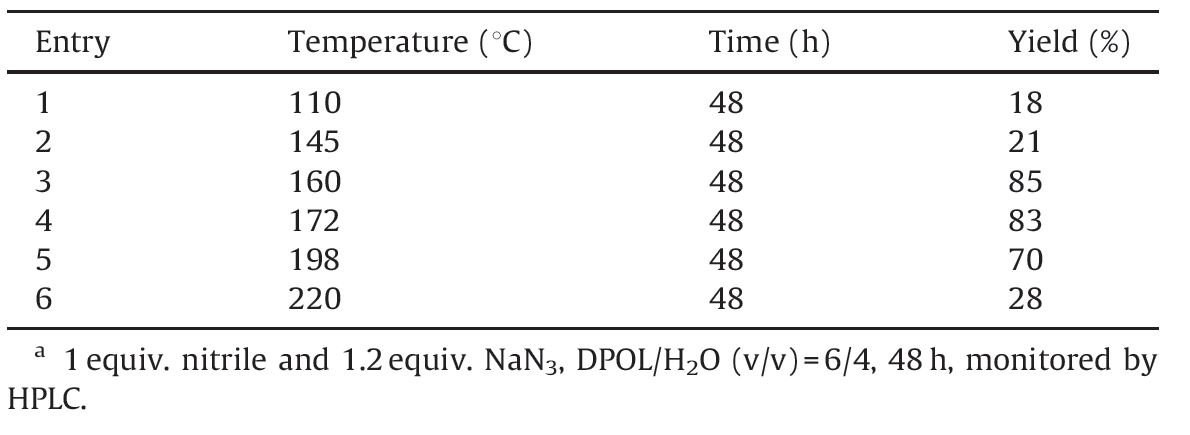
Next,reaction temperatures could neither be too low nor too high; higher temperatures result in decompositions of tetrazoles, and lead to a low yield and difficulty in separation,while the reaction is not likely to occur under lower temperatures. A temperature of 165 ℃ gives the best result according to Table 2.
Third,ammonium chloride and ammonium fluoride as a mixed catalyst played a very important role in the reaction. Ammonium chloride is a good catalyst for the synthesis of tetrazole,1.5 equiv. of ammonium chloride vs NaN3 would keep the reaction solution weakly alkaline,with no toxic azide acid emitted during reaction process,and the yield increased by 20% by the use of 0.2 equiv. of ammonium fluoride vs NaN3. The reason why it can improve the yield is still under investigation. Moreover,the yield has no significant increase after 48 h,so the best reaction time is 48 h according to Fig. 1.
| Table 1 Optimization of mixed solvent.a |
| Table 2 Optimization of reaction temperature.a |
Fourth,in theory,the ratio of 4'-methylbiphenyl-2-carbonitrile and NaN3 = 1:1 should be the proper condition for the reaction,But the conversion rate is only 75%,after 48 h at 160 ℃ in the H2O/ DPOL (6/4,v/v) mixed solvent. In order to improve the conversion rate,we increased the use of NaN3 at first,the conversion rate can be up to 90% if 1.5 equiv. NaN3 were used. Another problem appeared,the excess NaN3 must be eliminated after the reaction is complete,4'-methylbiphenyl-2-carbonitrile still cannot be reacted completely and needed to be recovered. So we did the opposite, excess 4'-methylbiphenyl-2-carbonitrile was used,since it can be easily extracted from the reaction solution. The result was satisfactory,if 1.2 times 4'-methylbiphenyl-2-carbonitrile is used, the conversion rate can be up to 95%.
Lastly,if the reaction takes place in an opened reactor,the conversion rate is only about 20%. The reason might be sublimation of NH4Cl and escaping of HN3.
All in all,the best reaction protocol (procedure) is: under hydrothermal conditions at 165 ℃,1.2 equiv. of 4'-methylbiphenyl-2- carbonitrile react with 1 equiv. of sodium azide in a mix solvent of H2O/DPOL,with 1.5 equiv. of ammonium chloride and 0.2 equiv. of ammonium fluoride as catalyst. Plausible mechanism for synthesis of 5-substituted 1H-tetrazole is shown in Scheme 2.
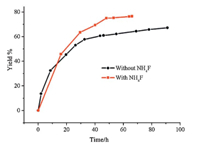
|
Download:
|
| Fig. 1. Reaction time and catalysis of NH4F. | |

|
Download:
|
| Scheme 2. Plausible mechanism for synthesis of MBPTZ. | |
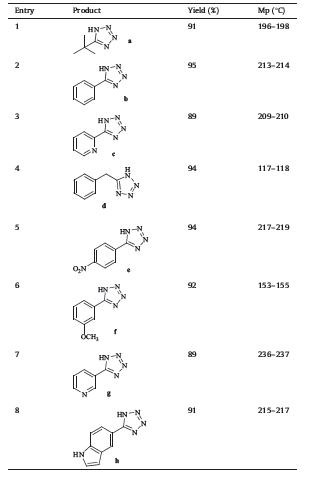
| Table 3 Tetrazole synthesis by hydrothermal conditions. |
With the optimal conditions in hand,we next examined the scope and limitation of this protocol (Table 3). It is notable that a wide range of functional groups,such as aryl,benzyl,and alkyl, were reactive under the conditions. IR,ESI-MS and 1H NMR data of the products is listed in Supporting information file.
The procedures have the following significant advantages in comparison with previously reported syntheses of tetrazole: (1) environmentally friendly,non-toxic ammonium chloride and ammonium fluoride instead of toxic metal ions act as catalyst; (2) Reaction process is more secure,sodium azide is more stable in the DPOL-water mixed solvent,the reaction liquid is alkaline,no toxic azide acid emitted; (3) The reaction is heated in a homogeneous solution,requiring a simple device,easy to expand production; (4) Simple post-processing,a product purity without recrystallization of up to 99%; (5) Production rate increased significantly,more than 95%; (6) Excess reactants can be easily recovered and applied again; the solvent can be reused to reduce costs.
In summary,a clean and safe synthesis method of 5-(4'- methylbiphenyl-2-yl)-1H-tetrazole was found: under hydrothermal conditions,1.2 equiv. of 4'-methylbiphenyl-2-carbonitrile react with 1 equiv. of sodium azide in a mixed solvent of DPOL/ H2O,with 1.5 equiv. of ammonium chloride and 0.2 equiv. of ammonium fluoride as catalyst. The yield can be up to 95%,and the purity of the product is 99% without further recrystallization.
This work was supported by the National Natural Science Foundations of China (Nos. 21101026,20931002,20921004, 90922005 and 91022003).
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.04.041.
| [1] | L.J. Goossen, B. Melzer, Synthesis of valsartan via decarboxylative biaryl coupling, J. Org. Chem. 72(2007) 7473-7476. |
| [2] | D. Carini, J. Duncia, P. Aldrich, et al., Nonpeptide angiotensin Ⅱ receptor antagonists: the discovery of a series of N-(biphenylylmethyl)imidazoles as potent, orally active antihypertensives, J. Med. Chem. 34(1991) 2525-2547. |
| [3] | W.G. Finnegan, R.A. Henry, R. Lofquist, An improved synthesis of 5-substituted tetrazoles, J. Am. Chem. Soc. 80(1958) 3908-3911. |
| [4] | G. Qi, Y. Dai, γ-Fe2O3: a magnetic separable catalyst for synthesis of 5-substituted 1H-tetrazoles from nitriles and sodium azide, Chin. Chem. Lett. 21(2010) 1029-1032. |
| [5] | T.A. Salama, S.S. Elmorsy, Silicon mediated synthesis and selected transformations of β-chloroketones, Chin. Chem. Lett. 22(2011) 1171-1174. |
| [6] | K.P. Nandre, J.K. Salunke, J.P. Nandre, et al., Glycerol mediated synthesis of 5-substituted 1H-tetrazole under catalyst free conditions, Chin. Chem. Lett. 23(2012) 161-164. |
| [7] | K. Koguro, T. Oga, S. Mitsui, et al., Novel synthesis of 5-substituted tetrazoles from nitriles, Synthesis 6(1998) 910-914. |
| [8] | F. Khamooshi, A.R. Modarresi-Alam, Solvent-free preparation of arylaminotetrazole derivatives using aluminum(Ⅲ) hydrogensulfate as an effective catalyst, Chin. Chem. Lett. 21(2010) 892-896. |
| [9] | Z.P. Demko, K.B. Sharpless, Preparation of 5-substituted 1-htetrazoles from nitriles in water, J. Org. Chem. 66(2001) 7945-7950. |
| [10] | F. Himo, Z.P. Demko, L. Noodleman, K.B. Sharpless, Why is tetrazole formation by addition of azide to organic nitriles catalyzed by zinc(Ⅱ) salts? J. Am. Chem. Soc. 125(2003) 9983-9987. |
| [11] | S.J. Wittenberger, B.G. Donner, Dialkyltin oxide mediated addition of trimethylsilyl azide to nitriles. A novel preparation of 5-substituted tetrazoles, J. Org. Chem. 58(1993) 4139-4141. |
| [12] | D. Amantini, R. Beleggia, F. Fringuelli, et al., TBAF-catalyzed synthesis of 5-substituted-1H-tetrazoles under solventless conditions, J. Org. Chem. 69(2004) 2896-2898. |
| [13] | S. Boris, M. Daniela, K. Daniel, Safe and fast tetrazole formation in ionic liquids, Tetrahedron 63(2007) 492-496. |
| [14] | R. Shelkar, A. Singh, J. Nagarkar, Amberlyst-15 catalyzed synthesis of 5-substituted-1H-tetrazole via [3+2] cycloaddition of nitriles and sodium azide, Tetrahedron Lett. 54(2013) 106-109. |
| [15] | H. Zhao, Z.R. Qu, H.Y. Ye, R.G. Xiong, In situ hydrothermal synthesis of tetrazole coordination polymers with interesting physical properties, Chem. Soc. Rev. 37(2008) 84-100. |
| [16] | Q. Ye, X.S. Wang, H. Zhao, R.G. Xiong, Highly stable olefin-Cu(Ⅰ) coordination oligomers and polymers, Chem. Soc. Rev. 34(2005) 208-225. |