Diarylimidazolium salts are well-known as the precursors of Nheterocyclic carbenes (NHCs)[1] and the skeleton of functional materials [2]. These compounds are usually synthesized by multistep ring closing methods starting from arylamines [3]. A classic example is the cyclic condensation using glyoxal,two equivalents of arylamine,and one equivalent of paraformaldehyde in the presence of hydrochloric acid [4].Recently,we discovered that Narylimidazoles could be quaternized by diaryliodonium salts under the catalysis of a copper salt [5]. This aryl quaternization methodology provides a direct and efficient route for the synthesis of diarylimidazolium salts. However,N-substituted imidazoles need to be prepared in advance [6].
Diaryliodonium salt,known for its ‘‘hyperleaving group ability’’ [7] of the aryliodanyl group (ArI),is a powerful arylating agent. It has previously been reported that diaryliodonium salts can react with oxygen,sulfur and nitrogen nucleophiles in the presence of a base to produce diaryl ethers,thioethers and arylamines [7e]. We therefore envisaged that 1H-imidzole itself may act as a base to facilitate the arylation of the N1 in the imidazole by a diaryliodonium salt and the in situ generated Narylimidazolewill be quaternized at the N3 position thereafter by the rest of the diaryliodonium salt to form a diarylimidazolium salt in one reaction flask. Herein,we want to report a direct synthesis of diarylimidazolium salts from 1H-imidzole using this one-pot strategy (Scheme 1).
A flame-dried Schlenk tube was charged with imidazole (0.25 mmol),a diaryiodonium salt (0.55 mmol),a copper salt (5 mol%) and a solvent (1 mL) with a stirring bar under an N2 atmosphere. The reaction mixture was stirred and heated for 16 h in an oil bath. After the mixture was cooled to room temperature, the solvent was removed under reduced pressure and the residue was passed through a silica gel column to afford the desired imidazolium salt. Analytical data for all new compounds and copies of 1H NMR and 13C NMR spectra can be found in Supporting information.
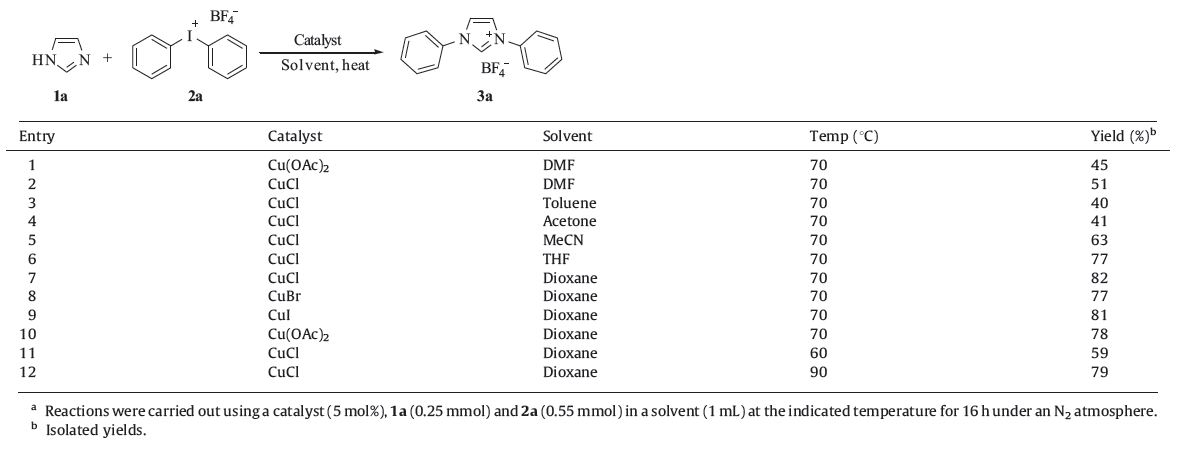
The reaction between 1H-imidazole and diphenyliodonium tetrafluoroborate salt (Ph2IBF4,2a) was performed first using our previous catalytic system (Cu(OAc)2 in DMF,at 70 ℃ under a nitrogen atmosphere (Table 1)). The tandem reaction did occur but the desired diphenylimidazolium salt 3a was obtained in only 45% yield after column chromatography purification (Table 1,entry 1). When CuCl was used instead,the yield of 3a was slightly improved to 51% (Table 1,entry 2). A range of solvents such as toluene, acetone,acetonitrile,tetrahydrofuran and dioxane were then screened and dioxane was found to be the best solvent with a yield of 82% (Table 1,entries 3-7). The other copper catalysts such as CuBr,CuI and Cu(OAc)2 also gave similar but slightly diminished yields (Table 1,entries 8-10). Lowering the reaction temperature to 60 ℃ significantly lowered the yield (Table 1,entry 11). Elevating the temperature to 90 ℃ showed no improvement of the yield (Table 1,entry 12). Thus,the optimized reaction conditionswere set to be 5 mol% CuCl in dioxane at 70 ℃ for 16 h,under which 3a was obtained in 82% isolated yield (Table 1,entry 7).
| Table 1 Optimization of the reaction conditions.a |

|
Download:
|
| Scheme 1. One-pot synthesis of diarylimidazolium salts. | |
With the optimal conditions in hand,a variety of representative diaryliodonium salts were used to react with 1H-imidazole 1a to directly construct imidazolium salts as illustrated in Scheme 2. Compared with unsubstituted diphenyliodonium salt 2a,substituted diphenyliodonium salts all gave lower yields of the corresponding imidazolium salts. With electron-donating methoxy substituent,di-p-methoxyphenylimidazolium salt 3ab was obtained in 73% yield,while with electron-withdrawing bromide substituent,di-p-bromophenylimidazolium salt 3ac was onlyobtained in 41% yield. The different reactivity between electrondonating and electron-withdrawing substituted diaryliodonium salts is in accordance with our previous findings in the aryl quaternization of N-substituted imidazoles. Two 2,5-dimethylphenyl groups could be added onto 1H-imidazole simultaneously to form sterically hindered di-(2,5-dimethylphenyl)imidazolium salt 3ad in 58% yield. However,even more hindered dimesitylimidazolium salt could not be obtained using this one-pot strategy because the N-arylation of the N1 in 1H-imidazole was unsuccessful. N-Mesitylimidazole is usually synthesized by a cyclization method starting from 2,4,6-trimethylaniline [8]. The sequential quaternization by dimesityliodonium salt could afford dimesitylimidazolium salt in a satisfactory yield following our previously reported procedure [5]. Diheteroarylimidazolium salts are suitable substrates for the one-pot synthesis. For example, di(2-thienyl)imidazolium salt 3ae was obtained in 75% yield after 24 h of reaction of 1H-imidazole with di(2-thienyl)iodonium salt.

|
Download:
|
| Scheme 2. Synthesis of azolium salts by symmetric diaryliodonium salts. General conditions: 1 (0.25 mmol),2 (0.55 mmol),and CuCl (5 mol%) in dioxane (1 mL) solution at 70 ℃ for 16 h (3ab and 3ae: 70 for 24 h; 3ac and 3cc: 100 ℃ for 24 h) under an N2 atmosphere. The yields are isolated yields. | |

|
Download:
|
| Scheme 3. Synthesis of azolium salts by unsymmetric diaryliodonium salts. General conditions: 1 (0.25 mmol),4 (0.55 mmol),and CuCl (5 mol%) in dioxane (1 mL) solution at 70 ℃ for 16 h under an N2 atmosphere. The yields are isolated yields. | |
Besides 1H-imidazole 1a,1H-benzimidazole 1b and 1H-1,2,4- triazole 1c could also be transformed into the corresponding azolium salts by reacting with a diaryliodonium salt in one single step. As illustrated in Scheme 2,diarylbenzimidazolium and triazolium salts 3ba-3cc were successfully obtained in 38%-69% yields,respectively. In comparison with the yields of imidazoliumsalts,the yields of diaryl benzimidazoliumand triazoliumsaltswere relatively low,which was probably due to the weaker basicity,or nucleophilicity of 1H-benzimidazole and 1H-1,2,4-triazole.
The unsymmetrical diaryliodonium salts are also applicable in this one-pot strategy. When [Mes-I-Ph]OTf reacted with 1Himidazole 1a,1H-benzimidazole 1b and 1H-1,2,4-triazole 1c, diphenyl imidazolium,benzimidazolium and triazolium salts were exclusively formed in 45%-58% yields,respectively (Scheme 3, 3aa’-3ca’). Di-heteroaryl imidazolium could also be synthesized using the [Ar-I-Mes] salts 4: di(2-thienyl)imidazolium salt 3ae’ was obtained in 42% yield by arylating 1H-imidazole 1a with [Mes- I-(2-thienyl)] salt. No arylation with the mesityl group was observed in any case mentioned above,suggesting that the bulky mesityl group could serve as a ‘‘dummy ligand’’ [7c, 9] in this reaction. The exclusive transfer of the less sterically hindered aryl group in the [Ar-I-Mes] salt is very useful when the symmetrical diaryliodonium salt is not readily available.
The double arylation of imidazole possibly proceeds through the nucleophilic attack at the Cu(III) center firstly by imidazole [7c], followed by the re-attack at the Cu(III) center by the in situ generated N-arylimidazole to afford the corresponding diarylimidazolium salt [5]. Imidazole itself acts as a base to facilitate thefirst catalytic cycle.
In summary,a one-pot synthesis of diaryimidazolium salts was achieved by using symmetrical and unsymmetrical diaryliodonium salts for a tandem arylation of 1H-imidazole in the presence of a copper catalyst. 1H-Benzimidazole and 1H-1,2,4-triazole are suitable substrates as well to form the corresponding diarylazolium salts. The present methodology provides a direct and convenient route to construct various diarylazolium salts.
This work was financially supported by the NSFC (Nos. 20902063,21172159,21025205 and 21021001) and the SRF for ROCS,SEM (No. 20111568-8-2). We thank the Centre of Testing & Analysis,Sichuan University for NMR measurements.
| [1] | (a) W.A. Herrmann, N-Heterocyclic carbenes: a new concept in organometallic catalysis, Angew. Chem. Int. Ed. 41(2002) 1290-1309;(b) S. Bellemin-Laponnaz, E. Despagnet-Ayoub, S. Díez-González, et al. Topics in Organometallic Chemistry, Vol. 21, Springer, Berlin,2007. |
| [2] | (a) A.J. Boydston, P.D. Vu, O.L. Dykhno, et al., Modular fluorescent benzobis(imidazolium) salts: syntheses, photophysical analyses, and applications, J. Am. Chem. Soc. 130(2008) 3143-3156;(b) R. Giernoth, Ionic liquids with a twist: new routes to liquid salts, Angew. Chem. Int. Ed. 49(2010) 5608-5609;(c) J.S. Park, E. Karnas, K. Ohkubo, et al., Ion-mediated electron transfer in a supramolecular donor-acceptor ensemble, Science 329(2010) 1324-1327;(d) J.T. Hutt, J. Jo, A. Olasz, et al., Fluorescence switching of imidazo[1,5-a]pyridinium ions: pH-sensors with dual emission pathways, Org. Lett. 14(2012) 3162-3165. |
| [3] | L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin-Laponnaz, V. César, Synthetic routes to N-heterocyclic carbene precursors, Chem. Rev. 111(2011) 2705-2733. |
| [4] | (a) A.J. Arduengo ??, Preparation of 1,3-disubstituted imidazolium salts, U.S. Patent 5,077,414, 1991.(b) A.J. Arduengo ??, R.L. Harlow, M. Kline, A stable crystalline carbene, J. Am. Chem. Soc. 113(1991) 361-363. |
| [5] | T. Lv, Z. Wang, J. You, J. Lan, G. Gao, Copper-catalyzed direct aryl quaternization of N-substituted imidazoles to form imidazolium salts, J. Org. Chem. 78(2013) 5723-5730. |
| [6] | (a) L. Zhu, L. Cheng, Y. Zhang, R. Xie, J. You, Highly efficient copper-catalyzed Narylation of nitrogen-containing heterocycles with aryl and heteroaryl halides, J. Org. Chem. 72(2007) 2737-2743;(b) L. Zhu, G. Li, L. Luo, P. Guo, J. Lan, J. You, Highly functional group tolerance in copper-catalyzed N-arylation of nitrogen-containing heterocycles under mild conditions, J. Org. Chem. 74(2009) 2200-2202. |
| [7] | (a) V.V. Zhdankin, P.J. Stang, Recent developments in the chemistry of polyvalent iodine compounds, Chem. Rev. 102(2002) 2523-2584;(b) V.V. Zhdankin, P.J. Stang, Chemistry of polyvalent iodine, Chem. Rev. 108(2008) 5299-5358;(c) E.A. Merritt, B. Olofsson, Diaryliodonium salts: a journey from obscurity to fame, Angew. Chem. Int. Ed. 48(2009) 9052-9070;(d) M.S. Yusubov, A.V. Maskaev, V.V. Zhdankin, Iodonium salts in organic synthesis, Arkivoc 1(2011) 370-409;(e) Y. Kita, G.F. Koser, M. Ochiai, et al. Topics in Current Chemistry, Vol. 224, Springer, Berlin,2003. |
| [8] | M.G. Gardiner, W.A. Herrmann, C.P. Reisinger, J. Schwarz, M. Spiegler, Dicationic chelating N-heterocyclic carbene complexes of palladium: new catalysts for the copolymerisation of C2H4 and CO, J. Organomet. Chem. 572(1999) 239-247. |
| [9] | N.R. Deprez, M.S. Sanford, Reactions of hypervalent iodine reagents with palladium: mechanisms and applications in organic synthesis, Inorg. Chem. 46(2007) 1924-1935. |