2. 南京大学 化学化工学院 生命分析化学国家重点实验室, 南京 210023;
3. 黑龙江省农垦乳品检测中心, 哈尔滨 150078
2. State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China;
3. Ministry of Agriculture Dairy Quality Supervision and Testing Center, Harbin 150078, China
喹诺酮类药物(fluoroquinolones,FQs)是一类具有6-氟-7-哌嗪-4-诺酮环结构(母核结构)的抗菌药物。为了控制和治疗动物疾病,养殖和治疗动物过程中会有多个喹诺酮类药物同时或交替使用的情况,这就导致了药物多个残留的发生。动物性食品中的喹诺酮类药物残留超标对人体存在潜在的威胁,这类兽药残留问题越来越被人们关注[1]。
氟喹诺酮类药物的国内外残留研究方法主要有微生物检测法[2]、色谱法[3-16]、免疫分析法[17-27]。其中色谱法包括高效液相色谱法(HPLC)[3-7]、高效液相色谱-荧光法(HPLC-FLD)[8-12]、液-质联用分析法(LC-MC)[13-16]等,免疫分析检测法包括胶体金试纸条法[22-23]、酶联免疫吸附法(ELISA)[17-21]、免疫荧光法[24]、化学发光免疫法[25]、蛋白芯片法[26-27]等。微生物法的优点是操作简便、成本低、检测快速,但该法的检测限量高于各国所规定的最低检测限量,灵敏度不高,特异性差;色谱法虽然检测结果精确,但是存在技术操作复杂、检测时间长、前处理烦锁、设备昂贵及费用高等缺点,所以不适于大规模的现场快速检测;免疫法中胶体金试纸条技术和ELISA作为一种方便、低成本、快速、高通量的筛选手段,广泛应用于众多领域,但是胶体金试纸条技术容易出现高的假阳性率或假阴性率,ELISA只能检测单组分残留;蛋白芯片法特异性强,反应灵敏,可实现大量样品的多组分残留快速检测,在国内外的研究中被广泛应用于食品监测[28-31]、临床监测[32-42]、药物筛选[41-42]等领域。随着科技的迅猛发展,蛋白芯片法将有可能取代酶联免疫吸附法和胶体金试纸条法,成为快速检测最常用的方法。
1 材料与仪器 1.1 材料喹诺酮类单克隆抗体(氧氟沙星、洛美沙星、培氟沙星、依诺沙星、诺氟沙星、恩诺沙星、环丙沙星、萘啶酸、氟甲喹)、纳米银标记的喹诺酮类人工抗原(氧氟沙星、洛美沙星、培氟沙星、依诺沙星、诺氟沙星、恩诺沙星、环丙沙星、萘啶酸、氟甲喹)、喹诺酮类对照品(氧氟沙星、洛美沙星、培氟沙星、依诺沙星、诺氟沙星、恩诺沙星、环丙沙星、萘啶酸、氟甲喹,含量≥99.8%)、纳米银增强显色液(显色液A和显色液B)等均由南京祥中生物科技有限公司提供;氯化钠、氯化钾、磷酸氢二钠(Na2HPO4·12H2O)、磷酸二氢钾(KH2PO4)、吐温20、乙二胺四乙酸(EDTA)均为分析纯,购买于南京化学试剂有限公司。牛血清白蛋白(BSA)购买于Sigma公司;实验所用的水均为18.25 MΩ·cm-1的超纯水,由实验室Milli-Q水系统生成;不同品牌的牛奶购买于当地超市。
1.2 仪器设备生物芯片点样仪、Q-array2000可视化生物芯片分析仪、微孔板恒温振荡仪、多管涡旋混旋仪, 南京祥中生物科技有限公司;Milli-Q纯水仪, 默克密理博公司;单道可调移液器, 苏州百得实验室仪器有限公司;KQ218型超声波清洗器, 昆山市超声仪器有限公司;微孔板, 南京祥中生物科技有限公司。
2 实验方法 2.1 检测原理在96孔板底部固定9个喹诺酮抗体,加入样品和被标记的抗原,标记的抗原和样品与固定的抗体进行特异性竞争结合。洗去未被结合的标记抗原和样品后,再加入显色液进行显色,显色液被标记的抗原催化显色。随着样品浓度增高,被标记的抗原和固定抗体结合的就越少,信号值就越低。所以样品浓度和检测信号值呈负相关。检测原理示意图见图 1。
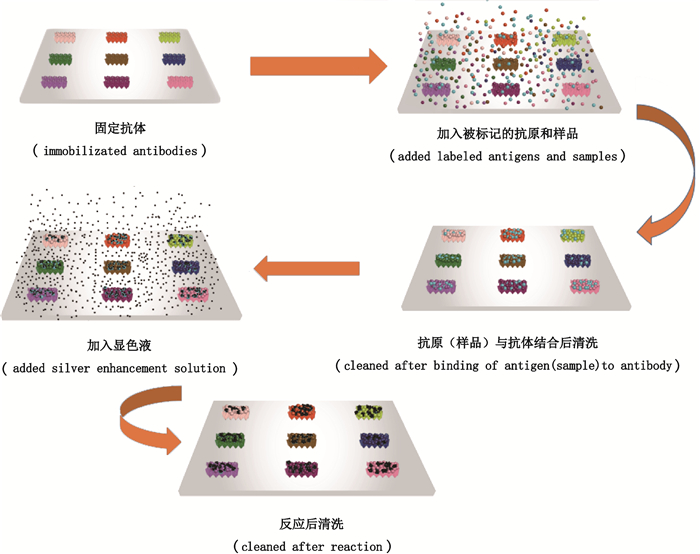
|
图 1 可视化蛋白芯片法同时检测喹诺酮类抗生素的检测原理示意图 Fig.1 Simultaneous detection of quinolones antibiotic with visual protein chip |
10 mmol·L-1磷酸盐缓冲液(pH 7.2):称取KH2PO4 0.2 g,Na2HPO4·12H2O 2.9 g、氯化钠8.0 g、氯化钾0.2 g,加水至1 000 mL。配制对照品溶液用。
洗涤缓冲液:在1 L磷酸盐缓冲液(pH 7.2)中加入500 μL吐温20,即得0.05%吐温20的磷酸盐缓冲液。
抗原抗体稀释液:用10 mmol·L-1磷酸盐缓冲液溶解BSA,使BSA终浓度为1 mg·mL-1。
封闭剂:用10 mmol·L-1磷酸盐缓冲液溶解BSA使其终浓度为10 mg·mL-1。
牛奶样品稀释液:在洗涤缓冲液中加入EDTA使其终浓度为1 mmol·L-1。
所有缓冲液在使用前通过0.22 μm孔径过滤器过滤。
2.3 可视化蛋白芯片的制备用生物芯片点样仪在96微孔板上制备6×5矩阵,分别按顺序点9个喹诺酮类抗体(氧氟沙星、洛美沙星、培氟沙星、依诺沙星、诺氟沙星、恩诺沙星、环丙沙星、萘啶酸、氟甲喹),点样量为50 nL·点-1,每样分别重复3个点。点样结束后,将芯片放于37 ℃恒温箱里孵育2 h,使抗体固定于板底。每孔加入200 μL芯片封闭剂,放于37 ℃恒温箱里孵育2 h,然后用洗涤缓冲液洗涤3次,每次10 s,拍干,置于4 ℃冰箱待用。
2.4 检测步骤 2.4.1 加样反应在制备好的孔板内每孔分别加入25 μL不同浓度混合对照品溶液(见表 3)(氧氟沙星、洛美沙星、培氟沙星、依诺沙星、诺氟沙星、恩诺沙星、环丙沙星、萘啶酸、氟甲喹)或样品,每个对照品或样品重复2个孔。每孔再加入25 μL标记后的喹诺酮类混合抗原,贴上盖板膜。于37 ℃恒温孔板振荡仪里孵育30 min,然后用洗涤液洗涤3次,每次15 s,拍干。
2.4.2 显色每孔加入银增强显色液(显色液A和显色液B按体积比1:1混合,现配现用)50 μL,放入37 ℃恒温孔板振荡仪孵育12 min,然后用超纯水洗3次,每次15 s,拍干。
2.4.3 结果处理用可视化生物芯片分析仪获取图像,并利用芯片分析软件进行结果处理分析。
3 结果与分析 3.1 抗体抗原条件优化抗原和抗体分别用抗原抗体稀释液进行稀释。氧氟沙星、洛美沙星、培氟沙星、依诺沙星抗体分别按1:5、1:10、1:20的比例稀释,诺氟沙星,环丙沙星,萘啶酸,氟甲喹抗体分别按1:1,1:2,1:4的比例稀释,恩诺沙星抗体分别按1:10,1:20,1:30的比例稀释。标记的喹诺酮类抗原分别按1:16,1:32,1:64,1:128的比例稀释。结果表明氧氟沙星、洛美沙星、培氟沙星、依诺沙星的最佳固定抗体浓度为1:10,诺氟沙星、环丙沙星、萘啶酸,氟甲喹的最佳固定抗体浓度为1:2,恩诺沙星最佳固定抗体浓度为1:30。经过优化后喹诺酮类抗原最终选择氧氟沙星1:32稀释,洛美沙星1:32稀释,萘啶酸1:128稀释,氟甲喹1:32稀释混合抗原。结果如表 1所示。
|
|
表 1 喹诺酮类抗体对应的结合抗原及相应信号 Tab.1 Quinolone antibodies corresponding with binding antigens and signals |
喹诺酮类抗体的特异性通常使用竞争抑制曲线来判断。使用不同浓度的抗原和干扰物计算其各自的结合比(B/B0:B代表不同浓度喹诺酮对照品下的信号值,B0代表不存在对照品时的信号值),绘制出竞争抑制曲线,并计算其各自的IC50。根据下列公式计算交叉反应率(CR)。
CR=[IC50(分析物)/IC50(干扰物)]×100%
IC50:分析物或干扰物诱导信号抑制50%所需的浓度。
结果表明,喹诺酮类抗体与9个喹诺酮药物交叉反应率均在1%~5%之间,如表 2所示。说明喹诺酮抗体的特异性好,相互间交叉反应率低,可以分别检测各个喹诺酮类抗生素。
|
|
表 2 喹诺酮类药物交叉反应率(%) Tab.2 Cross-reaction rates of quinolones |
探索喹诺酮类标准曲线采用间接竞争法。每个抗体浓度点3个平行微阵列点,信号值取其平均值。在一定范围内,随着竞争对照品浓度的增加,固定的抗体结合的人工抗原的含量会越来越少,因而检测到的信号值就会越低,超过这个范围后,检测信号不随竞争对照品浓度的增加而变化。用对照品氧氟沙星、洛美沙星、培氟沙星、依诺沙星、诺氟沙星、恩诺沙星、环丙沙星、萘啶酸、氟甲喹(如表 3所示)混合对照品溶液进行竞争抑制实验,图 2、3为同时测定9个喹诺酮类蛋白芯片竞争抑制后的扫描图。
|
|
表 3 喹诺酮类药物对照品质量浓度(ng·mL-1) Tab.3 mass concentration of quinolones reference substance |
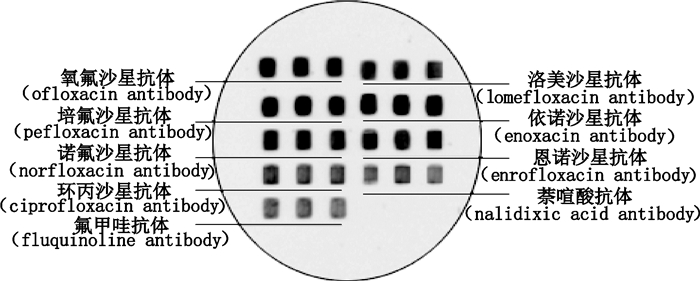
|
图 2 样品点样顺序示意图 Fig.2 Schematic of sample spot sequence |
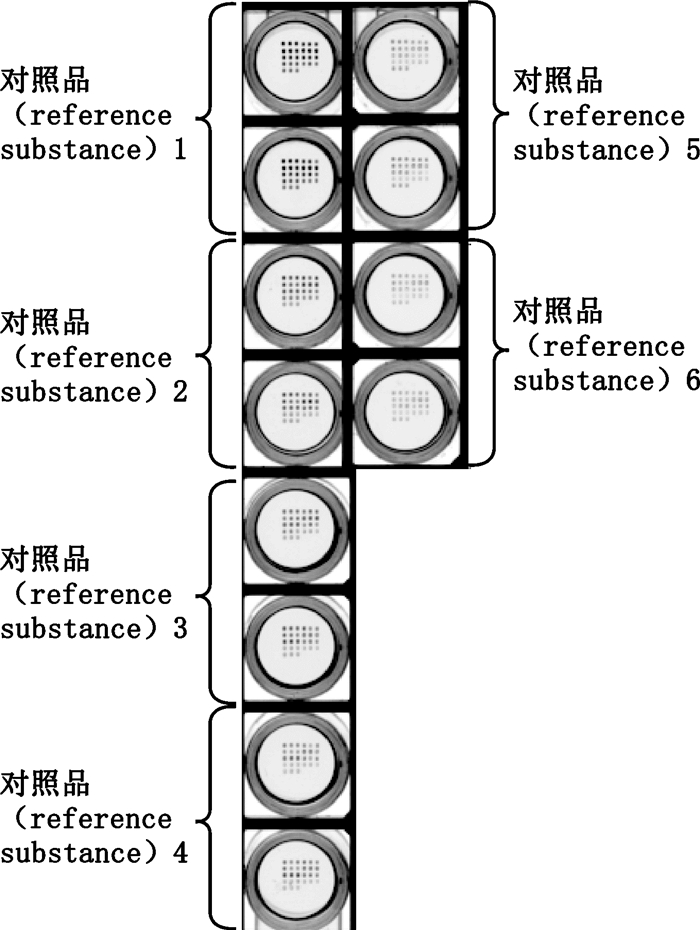
|
对照品1~6.同表 3 (same as Tab. 3) 图 3 不同浓度的对照品检测结果扫描图 Fig.3 Scanning results of different concentrations of reference subsfancos |
根据芯片分析软件提取的信号,对抑制率(B/B0)与浓度的对数(logC)作竞争抑制曲线。9个喹诺酮类药物的线性回归方程、相关系数、线性范围和检测下限如表 4所示。
|
|
表 4 喹诺酮类药物的线性回归方程、相关系数、线性范围 Tab.4 Linear regression equations, correlation coefficients and linear range of quinolones |
在优化的实验条件下,精确量取1 mL空白牛奶,分别添加对照品氧氟沙星、洛美沙星、培氟沙星、依诺沙星、诺氟沙星、恩诺沙星、环丙沙星、萘啶酸、氟甲喹,加标浓度如表 5所示,用样品稀释液1:1稀释后检测,每个样品做复孔,检测结果和加样顺序如图 4、表 6所示。每个样本重复测5次,喹诺酮类的回收率在70%~130之间,RSD%均在15%以内,可以满足喹诺酮类残留限量的要求。
|
|
表 5 喹诺酮类药物加标浓度 Tab.5 Additive concentration of quinolones |
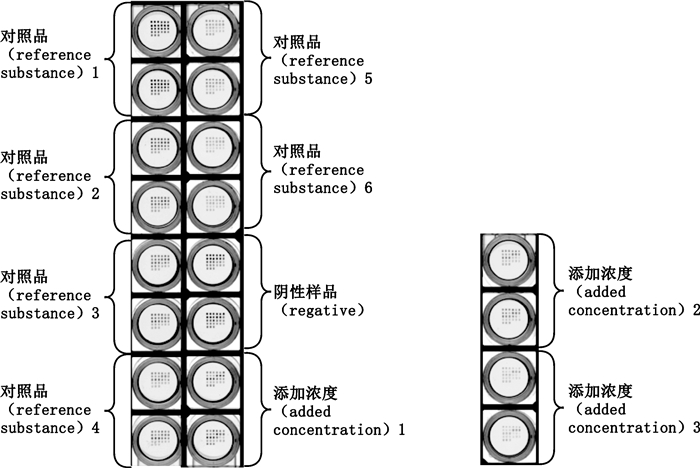
|
对照品1~6.同表 3 (same as Tab. 3) 图 4 牛奶中喹诺酮类药物加标检测结果扫描图 Fig.4 Scanning results of quinolones spiked in milk |
|
|
表 6 牛奶中喹诺酮类加标回收率 Tab.6 Recovery of quinolones in milk |
牛奶用样品稀释液1:1稀释后,按照“2.4”项下方法进行检测,结果见表 7。由表 7可得,从16个品牌牛奶中喹诺酮类抗生素的含量均小于1 ng·mL-1,符合国家规定的最大允许残留量。
|
|
表 7 牛奶样品中喹诺酮类药物含量测定结果(ng·mL-1,n=3) Tab.7 Contents of quinolones in milk samples |
本实验采用可视化蛋白芯片法同时测定牛奶中9个喹诺酮药物的残留,该实验采用固定抗体,标记抗原的方法,极大地减短了检测时间,提高了灵敏度,实验操作也很简单。同时对超市购得的16个品牌牛奶样品中喹诺酮类抗生素的含量进行了检测,均小于1 ng·mL-1,符合国家规定的最大允许残留量。本实验可同时检测对于喹诺酮类9个抗生素,提高了检测效率,对喹诺酮类多项药物残留的方法研究具有重要意义,具有很好的应用推广价值,为食品中氟喹诺酮类的残留检测提供一定的参考。
| [1] |
AHMAD N, JAVAID A, SULAIMAN SAS, et al. Resistance patterns, prevalence, and predictors of fluoroquinolones resistance in multidrug resistant tuberculosis patients[J]. Brazil J Infect Dis, 2016, 20(1): 41. DOI:10.1016/j.bjid.2015.09.011 |
| [2] |
NAVRÁTILOVÁ P, VYHNÁLKOVÁ J, VORLOVÁ L. Detection of fluoroquinolone residues in milk using Yersinia spp.strains:towards better sensitivity for flumequine determination[J]. J Vet Res, 2017, 61: 467. DOI:10.1515/jvetres-2017-0061 |
| [3] |
PATYRA E, KOWALCZYK E, GRELIK A, et al. Screening method for the determination of tetracyclines and fluoroquinolones in animal drinking water by liquid chromatography with diode array detector[J]. Pol J Vet Sci, 2015, 18(2): 283. DOI:10.1515/pjvs-2015-0037 |
| [4] |
D, ANGELO V, TESSARI F, BELLAGAMBA G, et al. Microextraction by packed sorbent and HPLC-PDA quantification of multiple anti-inflammatory drugs and fluoroquinolones in human plasma and urine[J]. J Enzym Inhib Med Chem, 2016, 31(Sup3): 110. DOI:10.1080/14756366.2016.1209496 |
| [5] |
WANG Q, WANG Y, ZHANG Z, et al. Waxberry-like magnetic porous carbon composites prepared from a nickel-organic framework for solid-phase extraction of fluoroquinolones[J]. Microchim Acta, 2017, 184(10): 4107. DOI:10.1007/s00604-017-2438-2 |
| [6] |
WANG GN, YANG K, LIU HZ, et al. Molecularly imprinted polymer-based solid phase extraction combined high performance liquid chromatography for determination of fluoroquinolones in milk[J]. Anal Methods-Uk, 2016, 8(27): 5511. DOI:10.1039/C6AY00810K |
| [7] |
WANG N, WANG Y, OMER AM, et al. Fabrication of novel surface-imprinted magnetic graphene oxide-grafted cellulose nanocrystals for selective extraction and fast adsorption of fluoroquinolones from water[J]. Anal Bioanal Chem, 2017, 409(28): 6643. DOI:10.1007/s00216-017-0619-9 |
| [8] |
SEYHAN BOZKURT S, ERDOGAN D, ANTEP M, et al. Use of ionic liquid based chitosan as sorbent for preconcentration of fluoroquinolones in milk, egg, fish, bovine, and chicken meat samples by solid phase extraction prior to HPLC determination[J]. J Liquid Chromatogr Related, 2016, 39(1): 21. DOI:10.1080/10826076.2015.1116010 |
| [9] |
KARAMI-OSBOO R, SHOJAEE MH, MIRI R, et al. Simultaneous determination of six fluoroquinolones in milk by validated QuEChERS-DLLME HPLC-FLD[J]. Anal Methods, 2014, 6(15): 5632. DOI:10.1039/C4AY00848K |
| [10] |
SUN X, WANG J, LI Y, et al. Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples[J]. J Chromatogr A, 2014, 1359: 1. DOI:10.1016/j.chroma.2014.07.007 |
| [11] |
NEVES M, SILVA G, BRITO N, et al. Aqueous ultrasound-assisted extraction for the determination of fluoroquinolones in mangrove sediment by high-performance liquid chromatography and fluorescence detector[J]. J Brazil Chem Soc, 2018, 29(1): 24. |
| [12] |
VAKH C, ALABOUD M, LEBEDINETS S, et al. An automated magnetic dispersive micro-solid phase extraction in a fluidized reactor for the determination of fluoroquinolones in baby food samples[J]. Anal Chim Acta, 2018, 1001: 59. DOI:10.1016/j.aca.2017.11.065 |
| [13] |
CHEN L, HUANG X. Sensitive Monitoring of Fluoroquinolones in Milk and Honey Using Multiple Monolithic Fiber Solid-Phase Microextraction Coupled to Liquid Chromatography Tandem Mass Spectrometry[J]. J Agric Food Chem, 2016, 64(45): 8684. DOI:10.1021/acs.jafc.6b03965 |
| [14] |
BARRETO F, RIBEIRO CBD, HOFF RB, et al. Development and validation of a high-throughput method for determination of nine fluoroquinolones residues in muscle of different animal species by liquid chromatography coupled to tandem mass spectrometry with low temperature clean up[J]. J Chromatogr A, 2017, 1521: 131. DOI:10.1016/j.chroma.2017.09.036 |
| [15] |
JANUSCH F, SCHERZ G, MOHRING SAI, et al. Determination of fluoroquinolones in chicken feces-A new liquid-liquid extraction method combined with LC-MS/MS[J]. Environ Toxicol Pharm, 2014, 38: 792. DOI:10.1016/j.etap.2014.09.011 |
| [16] |
LUA X, ZHOU Y, ZHANG J, et al. Determination of fluoroquinolones in cattle manure-based biogas residue by ultrasonic-enhanced microwave-assisted extraction followed by online solid phase extraction-ultra-high performance liquid chromatography-tandem mass spectrometry[J]. Chromatogr B, 2018(1086): 166. |
| [17] |
LIU N, ZHAO Z, TAN Y, et al. Simultaneous Raising of Rabbit Monoclonal Antibodies to Fluoroquinolones with Diverse Recognition Functionalities via Single Mixture Immunization[J]. Anal Chem, 2015, 88(2): 1246. |
| [18] |
LIU YZ, ZHAO GX, WANG P, et al. Production of the broad specific monoclonal antibody against sarafloxacin for rapid immunoscreening of 12 fluoroquinolones in meat[J]. J Environ Sci Health B, 2013, 48(2): 139. DOI:10.1080/03601234.2013.727668 |
| [19] |
LI Y, JI B, CHEN W, et al. Production of new class-specific polyclonal antibody for determination of fluoroquinolones antibiotics by indirect competitive ELISA[J]. Food Agr Immunol, 2008, 19(4): 251. DOI:10.1080/09540100802471538 |
| [20] |
ZHANG B, DU D, MENG M, et al. A magnetic particle-based competitive enzyme immunoassay for rapid determination of ciprofloxacin:a potential method for the general detection of fluoroquinolones[J]. Anal Lett, 2014, 47(7): 1134. DOI:10.1080/00032719.2013.865197 |
| [21] |
CUI M, LIN H, WANG X, et al. 5-sulfosalicylic acid dihydrate-based pretreatment for the modification of enzyme-linked immunoassay of fluoroquinolones in fishery products[J]. J Immunoassay Immunochem, 2015, 36(5): 517. DOI:10.1080/15321819.2015.1006330 |
| [22] |
SUI J, LIN H, CAO L, et al. Dot-immunogold filtration assay for rapid screening of three fluoroquinolones[J]. Food Agric Immunol, 2009, 20(2): 125. DOI:10.1080/09540100902889936 |
| [23] |
ZHU Y, LI L, WANG Z, et al. Development of an Immunochromatography strip for the rapid detection of 12 fluoroquinolones in chicken muscle and liver[J]. J Agric Food Chem, 2008, 56: 5469. DOI:10.1021/jf800274f |
| [24] |
HU G, SHENG W, ZHANG Y, et al. A novel and sensitive fluorescence immunoassay for the detection of fluoroquinolones in animal-derived foods using upconversion nanoparticles as labels[J]. Anal Bioanal Chem, 2015, 407(28): 8487. DOI:10.1007/s00216-015-8996-4 |
| [25] |
TAO X, WANG J, XIE Y, et al. Dual-label chemiluminescence strategy for multiplexed immunoassay of 20 fluoroquinolones, 15β-lactams, 15 sulfonamides, and CAP in milk[J]. Food Anal Method, 2017, 10(9): 3009. DOI:10.1007/s12161-017-0865-7 |
| [26] |
钟文英, 王兴如, 许丹科, 等. 可视化蛋白芯片法同时检测牛乳中残留的磺胺类和喹诺酮类药物[J]. 食品科学, 2016(02): 193. ZHONG WY, WANG XR, XU DK, et al. Simultaneous determination of multiresidues of sulfonamides and quinolones in milk with visual protein chip[J]. Food Sci, 2016(2): 193. |
| [27] |
LI Z, LI Z, ZHAO D, et al. Smartphone-based visualized microarray detection for multiplexed harmful substances in milk[J]. Biosens Bioelectron, 2017, 87: 874. DOI:10.1016/j.bios.2016.09.046 |
| [28] |
王兴如, 钟文英, 李周敏, 等. 微孔板生物芯片测定蜂蜜中四环素残留方法的研究及应用[J]. 药物分析杂志, 2015, 35(7): 1240. WANG XR, ZHONG WY, LI ZM, et al. Visual microplate biochip for determination of tetracycline residues in honey[J]. Chin J Pharm Anal, 2015, 35(7): 1240. |
| [29] |
李周敏, 许丹科. 可视化蛋白芯片检测牛奶中庆大霉素的方法研究[J]. 分析科学学报, 2014, 20(5): 687. LI ZM, XU DK. Protein chips for visualized determination of gentamicin residues in milk[J]. J Anal Sci, 2014, 20(5): 687. |
| [30] |
LI Z, LI Z, JIANG J, et al. Simultaneous detection of various contaminants in milk based on visualized microarray[J]. Food Control, 2017, 73: 994. DOI:10.1016/j.foodcont.2016.10.009 |
| [31] |
LI Z, LI Z, XU D. Simultaneous detection of four nitrofuran metabolites in honey by using a visualized microarray screen assay[J]. Food Chem, 2017, 221: 1813. DOI:10.1016/j.foodchem.2016.10.099 |
| [32] |
LI Z, LI Z, NIU Q, et al. Visual microarray detection for human IgE based on silver nanoparticles[J]. Sens Actuat B:Chem, 2017, 239: 45. DOI:10.1016/j.snb.2016.07.142 |
| [33] |
SYAFRIZAYANTI, LUEONG SS, DI C, et al. Personalised proteome analysis by means of protein microarrays made from individual patient samples[J]. Sci RepUk, 2017, 7: 1. DOI:10.1038/s41598-016-0028-x |
| [34] |
SIEVERS S, CRETICH M, GAGNI P, et al. Performance of a polymer coated silicon microarray for simultaneous detection of food allergen-specific IgE and IgG4[J]. Clin Exp Allergy, 2017, 47(8): 1057. DOI:10.1111/cea.2017.47.issue-8 |
| [35] |
BELUSHKIN A, YESILKOY F, ALTUG H. Nanoparticle-enhanced plasmonic biosensor for digital biomarker detection in a microarray[J]. Acs Nano, 2018, 12(5): 4453. DOI:10.1021/acsnano.8b00519 |
| [36] |
QUAN X, DING Y, FENG R, et al. Expression profile of cytokines in gastric cancer patients using proteomic antibody microarray[J]. Oncol Lett, 2017, 14: 7360. |
| [37] |
OKUMA HS, KOIZUMI F, HIRAKAWA A, et al. Clinical and microarray analysis of breast cancers of all subtypes from two prospective preoperative chemotherapy studies[J]. Brit J Cancer, 2016, 115(4): 411. |
| [38] |
HARALAMBIEVA IH, GIBSON MJ, KENNEDY RB, et al. Characterization of rubella-specific humoral immunity following two doses of MMR vaccine using proteome microarray technology[J]. Plos One, 2017, 12(11): e188149. |
| [39] |
LIU L, WU S, JING F, et al. Bead-based microarray immunoassay for lung cancer biomarkers using quantum dots as labels[J]. Biosens Bioelectronics, 2016, 80: 300. DOI:10.1016/j.bios.2016.01.084 |
| [40] |
SAUER U. Analytical protein microarrays:advancements towards clinical applications[J]. Sensors-Basel, 2017, 17(2): 256. |
| [41] |
ZHOU W, YANG M, LI S, et al. Surface plasmon resonance imaging validation of small molecule drugs binding on target protein microarrays[J]. Appl Surf Sci, 2018, 450: 328. DOI:10.1016/j.apsusc.2018.04.072 |
| [42] |
LI Z, YAN M, LI Z, et al. A multiplexed screening method for agonists and antagonists of the estrogen receptor protein[J]. Anal Bioanal Chem, 2012, 403(5): 1373. DOI:10.1007/s00216-012-5933-7 |
 2019, Vol. 39
2019, Vol. 39 