2. Department of Geology, Juniata College, 1700 Moore St Huntingdon PA 16652;
3. 西藏自治区地质矿产勘查开发局第二地质大队, 拉萨 850007
2. Department of Geology, Juniata College, 1700 Moore St Huntingdon PA 16652, USA;
3. No.2 Geological Party, Bureau of Geology and Mineral Exploration and Development, Lhasa 850007, China
金属来源及其成矿过程是金属矿床研究以及找矿勘查中重点关注的基本核心问题。近代矿床学的许多重大进展,都与稳定同位素的示踪技术有关(王跃和朱祥坤, 2012)。过去,矿床学家一直借助于与金属矿物共生的脉石矿物的传统同位素(C-H-O-S-N、Sr-Nd等)地球化学研究示踪成矿流体、物质来源以及反演成矿过程,进而为找矿勘查提供理论依据。但是,从成矿学的角度来看,这些元素是矿化剂元素,并非成矿元素本身。因此,金属矿床传统同位素地球化学研究具有一定的间接性和猜测性(王跃和朱祥坤, 2010a, b, 2012)。
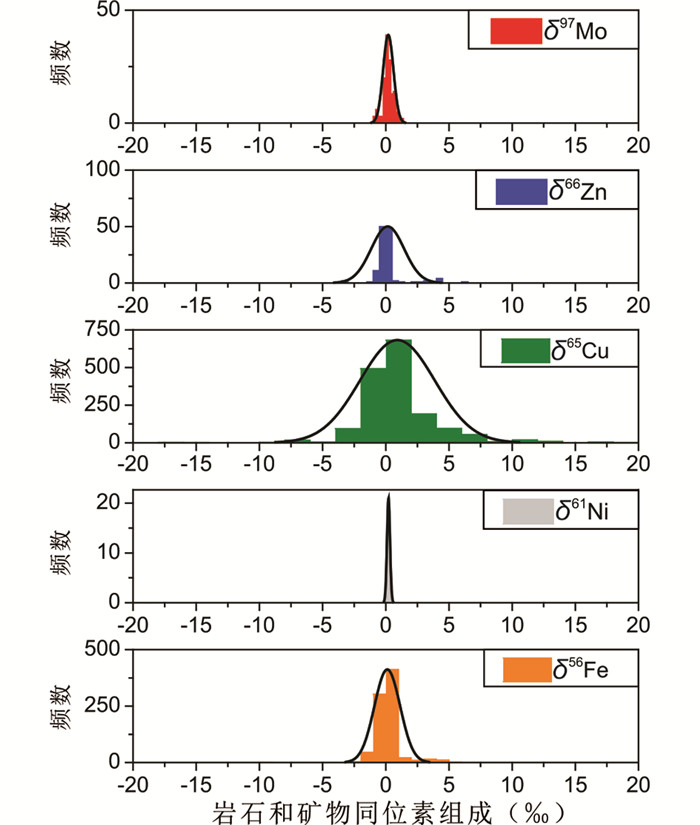
近年来,随着多接收电感耦合等离子质谱仪(MC-ICP-MS)的诞生以及相应测试技术的不断发展和提高(Maréchal et al., 1999; Belshaw et al., 2000),使得我们可以直接精确地测量自然物质的金属稳定同位素组成(Fe、Cu、Zn、Ni、Mo等;图 1),促使金属稳定同位素地球化学研究取得突破性进展(Maréchal and Sheppard, 2002; Zhu et al., 2002; Liu et al., 2014; Shafiei et al., 2015)。目前,越来越多的矿床学家开始尝试从金属元素同位素地球化学行为入手,直接示踪金属矿床成矿作用中成矿元素的源运储,还原金属矿床成矿过程,约束金属矿床成因,发展成矿理论,指导找矿勘查。经过众多学者多年来不断地尝试和不懈地努力,铁铜锌(Fe-Cu-Zn)等过渡金属稳定同位素在矿床学研究以及找矿勘查中已经得到了广泛地应用,并且在反演成矿过程、约束矿床成因以及指导找矿勘查等方面展现出良好的应用前景。
|
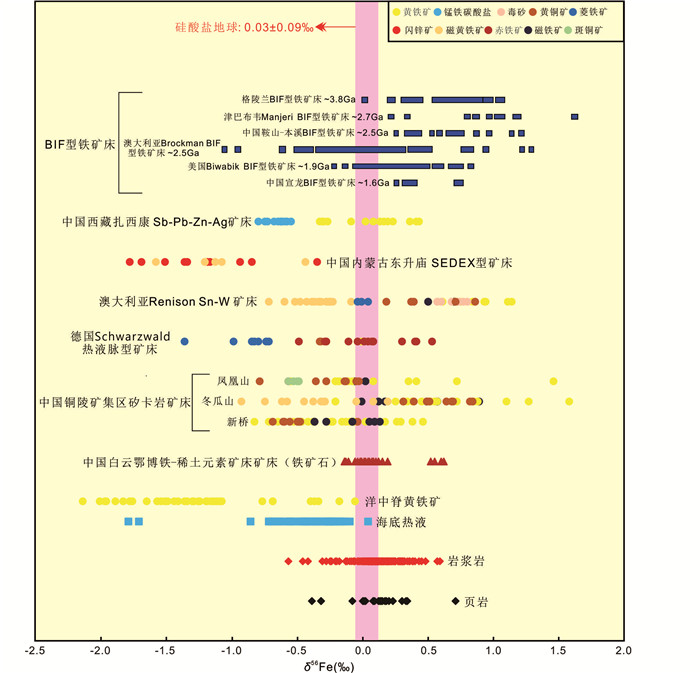
图 1 自然界岩石和矿物过渡金属同位素组成变化柱状图(据Mathur and Fantle, 2015修改) 所有数据均为相对于统一国际标样的同位素值(Mo: NIST SRM 3134; Zn: JMC Lyon; Cu: NIST 976; Ni: NIST 986; Fe: IRMM-014) Fig. 1 Variations of transition metal isotopic values in rocks and minerals (modified after Mathur and Fantle, 2015) All data are compared to the same international isotope standard (Mo: NIST SRM 3134; Zn: JMC Lyon; Cu: NIST 976; Ni: NIST 986; Fe: IRMM-014) |
本文系统统计和总结了世界范围内相关矿石、水、岩石、土壤和植物等自然物质的Fe-Cu-Zn同位素研究成果,以期展示其在矿床学研究以及找矿勘查中的潜在应用范围。
1 分析方法 1.1 Fe同位素Fe(铁)是地球上含量最高的变价元素,常见价态包括0,+2和+3;含铁矿物主要有黄铁矿、赤铁矿、菱铁矿、磁铁矿、针铁矿和铁绿泥石等。铁有四个稳定同位素,包括54Fe(5.84%)、56Fe(91.76%)、57Fe(2.12%)和58Fe(0.28%)(王跃和朱祥坤, 2012)。Fe同位素的δ形式表述如下:
δ56Fe (‰)=[(56Fe/54Fe)样品/(56Fe/54Fe)标样-1]×1000
δ57Fe (‰)=[(57Fe/54Fe)样品/(57Fe/54Fe)标样-1]×1000
ΔxFeA-B=δxFeA-δxFeB以及Δ56Fe=0.678Δ57Fe(质量相关分馏情况下)
Fe同位素的纯化是采用BioRad公司的AG-X和MP-1阴离子交换树脂来完成的,但是不同实验室的纯化方法中,把Fe从树脂中淋滤出来的步骤有几个细微差异。在Fe的离子交换柱分离过程中,有一点十分关键,就是要从基质中去除铬元素,因为铬会对54Fe造成直接干扰。Fe同位素测试必须使用质谱的高分辨模式来排除质量数56位置强大的氩氧干扰。用来上机分析的溶液浓度必须较高,通常在3×10-6~5×10-6左右。文献中质量数均使用峰的“右肩”位置,质量偏差采用间插矫正或使用铜元素以蒸发率进行矫正。铁有2个国际标样,分别是:(1)15个地球火成岩和5个月球高钛玄武岩构成的火成岩平均值;(2)欧洲委员会参考物质及测量协会提供的IRMM-014标准样品(Taylor et al., 1992)。2个标样间的转换关系是δ56Fe火成岩平均值=δ56FeIRMM-014-0.09‰,δ57Fe火成岩平均值=δ57FeIRMM-014-0.11‰ (Johnson et al., 2004)。本文所有数据均采用δ56Fe的形式并且相对于IRMM-014标样进行报道。
1.2 Zn-Cu同位素Zn(锌)和Cu(铜)都是自然界中普遍存在的过渡元素, 是矿床学研究中重要的成矿元素,也是生命活动所必需的微量营养元素。Zn在地质过程中主要以+2价离子形式出现,主要成矿矿物包括闪锌矿和菱锌矿,有64Zn(46.63%)、66Zn(27.90%)、67Zn(4.10%)、68Zn(18.75%)、70Zn(0.62%)等5个稳定同位素(王跃和朱祥坤, 2010a)。Cu通常呈0、+1和+2价,在自然界中广泛地赋存于岩石、矿物、河流和生物体中,参与多种地球化学和生物化学过程,主要矿物包括黄铜矿、辉铜矿、斑铜矿、黝铜矿、蓝铜矿、赤铜矿、黑铜矿、橄榄铜矿等,Cu有两个稳定同位素,分别为65Cu(30.826%)、63Cu(69.174%)(王跃和朱祥坤, 2010b)。
目前,Zn-Cu同位素测试技术已经相对比较成熟。分析样品中Cu同位素组成的测定方法有两种,一种是溶液法,一种是激光剥蚀法;而Zn同位素主要是采用溶液法进行分析。在溶液法中,除了天然铜以外,黄铜矿(CuFeS2)、辉铜矿(CuS2)、铜氧化物、以及闪锌矿(ZnS)都需要采用阴离子交换色谱分析法进行纯化以使铜和锌与复杂的基体分离,绝大多数研究采用的是BioRad MP-1阴离子交换树脂(Maréchal et al., 1999; Maréchal and Albarède, 2002; Zhu et al., 2002; Mason et al., 2004; Bermin et al., 2006; Chapman et al., 2006; Borrok et al., 2007; Moynier et al., 2007; Balistrieri et al., 2008; Peel et al., 2008; Petit et al., 2008; Pokrovsky et al., 2008; Vance et al., 2008; Li et al., 2009; Mathur et al., 2009, 2012; Pribil et al., 2009; Sossi et al., 2015)。在上述研究中,有一点至关重要,如果铜没有被完全回收,样品就会在树脂中产生分馏。由于树脂中元素的过饱和会使铜在化学洗脱流程之前就发生损失,因此在已知离子含量的情况下采用多根柱子分离铜,最后再将溶液合并的方法是必要的。目前仅有为数不多的几项研究尝试了用激光剥蚀法分析铜同位素(Jackson and Günther, 2003; Graham et al., 2004; Kuhn et al., 2007; Ikehata et al., 2008, 2011; Ikehata and Hirata, 2013),研究表明要想获得有意义的同位素数据,基质匹配十分重要。
对Zn-Cu同位素比值的质谱分析方法随三种主要的质谱品牌制造的质谱仪器(Neptune、Micromass Isoprobe和Nu)的不同而有所差异。在Cu同位素比值分析中仪器测试的相同点有:质谱采用低分辨模式、每一次测试中背景噪声都必须进行扣除、间插标样和样品间的浓度匹配必须在50%以内、不使用辅助气、至少要取得25个比值以确保较低的分析偏差。样品吸取速率、铜的浓度、HT设置、样品引入方式以及其他诸多不同都曾见相关文献报道。由于铜仅有两个同位素,无法对其质量相关分馏进行内部检验,因此对铜同位素的质量偏差矫正是非常重要的。Ni和Zn都曾被用于对Cu同位素测试的质量偏差矫正并且都获得了大约0.04‰(2σ,95%置信区间)的分析精度(Chapman et al., 2006; Liu et al., 2015; Peel et al., 2008; Sossi et al., 2015)。文献中均采用蒸发率来对样品和标样进行矫正,将获得的矫正值再使用在标样-样品-标样间插上。研究表明,不使用Ni和Zn进行矫正的话,分析误差大概会在0.12‰(2σ,95%置信区间)的水平。对Zn来说,可以使用应用在Cu同位素上的质量偏差矫正和质量相关分馏矫正方法进行监测,因为Zn有两个以上同位素。同质异位素干扰是使用任何质谱进行同位素测试中都会遇到的常见问题,对铜也不例外。常见的元素干扰来自于过量的Ti、Na和Mg,它们均会形成分子,干扰质量数在63或65位置的测试。曾有研究注意到过渡族金属与铜的比例超过10:1时会使铜同位素测试产生问题。
本文Cu同位素数据采用常用的千分表达方式:δ65Cu (‰)=[(65Cu/63Cu)样品/(65Cu/63Cu)NIST976-1]×1000)。最早对Cu同位素的报道采用的是万分位(ε值)的表达方式(Zhu et al., 2000, 2002),这是由于早期人们没有想到Cu同位素会有现今这么大的变化范围。大多数Cu同位素数据是基于NIST 976同位素标样进行报道,但是由于该标样已无法再买到,所以有些文章开发了新的标样并且相对NIST 976进行了标定以便和早期数据进行对比。迄今为止,国际上尚没有统一的Zn同位素标样。目前常见的Zn同位素标样有JMC-Lyon、IRMM-3702以及AA-ETH(Archer et al., 2017),这些标样之间的转换关系为δ66ZnAA-ETH=δ66ZnIRMM-3702=δ66ZnJMC 3-0749 L-0.28‰(Archer et al., 2017)。本文所有数据都采用δ66Zn的形式并且相对于AA-ETH标样进行报道:δ66Zn (‰)=[(66Zn/64Zn)样品/(66Zn/64Zn)标样-1]×1000。
2 Fe-Cu-Zn同位素在不同类型矿床研究及找矿勘查中的应用 2.1 高温成矿系统在高温成矿系统的相关研究中,Fe-Cu同位素的应用更为广泛,Fe同位素主要应用于岩浆型、岩浆热液型、矽卡岩型铁矿床;Cu同位素主要应用于层状镁铁质侵入体、斑岩型铜矿床、矽卡岩矿床和VHMS(VMS)型矿床研究;而Zn同位素则只见于VHMS(VMS)型和Irish型矿床的相关研究中。这可能与不同成矿体系中不同元素的成矿作用差异性有关,在金属稳定同位素研究中,我们应该尽量选择矿床中最主要的成矿元素的同位素进行研究。在高温成矿系统中,物理化学条件、氧化还原状态的改变会引起金属稳定同位素的瑞利分馏效应,致使Fe-Cu-Zn同位素在时间和空间尺度上产生系统的变化规律。我们可以利用Fe-Cu-Zn同位素的时空变化规律来反演成矿热液的时空演化模式、还原矿床形成过程、指示矿体延伸方向,进而为找矿勘查提供理论依据。此外,不同种类的矿床由于成矿作用的差异,Fe-Cu-Zn同位素组成也会具有明显的差别,可以作为判断矿床种类、约束矿床成因的有力证据。
2.1.1 岩浆型、岩浆热液型、矽卡岩型铁矿床——Fe同位素下述这些研究以矿石及其与相关岩浆岩的关系为重点研究对象,证实了Fe同位素在示踪金属来源和反演流体演化过程方面的应用潜力。目前为止,尚没有研究发现Fe同位素应用于找矿勘查的直接证据,但是这些研究显示早期和晚期形成的矿物具有明显不同的Fe同位素组成。
Sun et al. (2013)对中国内蒙古白云鄂博铁-稀土元素矿床进行了研究,该矿床为最高温的岩浆型铁矿床,研究结果显示矿石样品的Fe同位素分馏很小且δ56Fe值集中在0‰附近、Fe含量和δ56Fe值之间未表现出相关性,这些特征明显区别于其它成因的矿床:(1)沉积型Fe矿床:δ56Fe值重且变化范围大,δ56Fe值和Fe含量具有线性关系;(2)德国南部Schwarzward低温热液脉型多金属矿床以及新桥矽卡岩型铜硫铁矿床:δ56Fe值相对沉积型铁矿床轻且变化范围适中、不同阶段δ56Fe值变化明显(图 2、图 3);同时,根据测试所得数据,利用共生矿物对之间的δ56Fe差值Δ56Fe磁铁矿-白云石和Δ56Fe赤铁矿-磁铁矿进行理论计算,Fe同位素地质温度计计算结果显示白云鄂博铁-稀土元素矿床成矿温度在700~900℃。综合以上证据,排除了白云鄂博矿床的沉积成因和热液过程,最终确定白云鄂博矿床为岩浆成因。Zhu et al. (2016)对中国境内邯邢矽卡岩铁矿床相关样品进行了Fe同位素测试,发现如下规律:闪长岩样品具有非常明显的Fe同位素分馏(-0.07‰~0.21‰)、惰性元素含量变化很小(例如Si、Ti、Al、P等)、LOI和Fe2O3T之间存在负相关关系,据此作者推断闪长岩的Fe同位素变化与早期的岩浆过程和晚期的蚀变关系不大,矽卡岩化才是引起闪长岩Fe同位素变化的主要原因;同时,根据矿石矿物(0.07‰~0.48‰)和闪长岩Fe同位素组成的对比,排除了成矿物质来源于先前已存矿体和相关岩浆岩的可能,确定了该矿床的岩浆热液成因。Bilenker et al. (2016)研究了智利北部的铁氧化物-磷灰石矿床,这些矿床成因具有争议,主要观点包括岩浆或者低温来源;根据Fe同位素测试结果,磁铁矿样品的δ56Fe值(0.08‰~0.53‰)与高温岩浆或岩浆热液成因磁铁矿(0.06‰~0.49‰)以及南非Bushveld复式岩体中磁铁矿(0.28‰~0.86‰)的δ56Fe值接近,结合O同位素数据,推断这些铁氧化物-磷灰石矿床具有岩浆来源且高温岩浆过程是导致Fe同位素变化的主要原因。
|
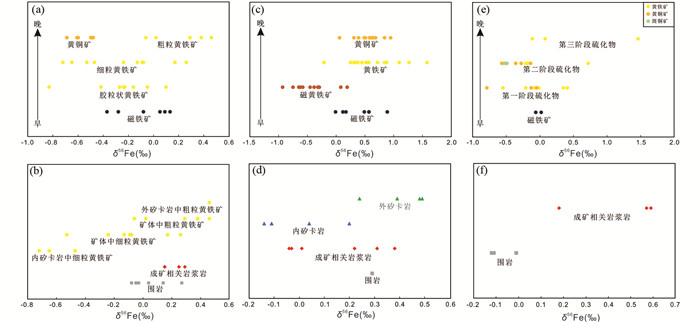
图 3 铜陵矿集区矽卡岩矿床Fe同位素变化图(据Wang et al., 2011, 2015修改) 新桥矽卡岩Cu-S-Fe-Au矿床δ56Fe值随时间变化趋势图(a)和空间变化趋势图(b);冬瓜山矽卡岩Cu-Au矿床δ56Fe值随时间变化趋势图(c)和空间变化趋势图(d);凤凰山矽卡岩Cu-Fe-Au矿床δ56Fe值随时间变化趋势图(e)和空间变化趋势图(f) Fig. 3 The Fe isotopic variations in the skarn deposits within Tongling ore district (modified after Wang et al., 2011, 2015) The temporal (a) and spatial (b) Fe isotopic variation in the Xinqiao skarn Cu-S-Fe-Au deposit; the temporal (c) and spatial (d) Fe isotopic variation in the Dongguashan skarn Cu-Au deposit; the temporal (e) and spatial (f) Fe isotopic variation in the Fenghuangshan skarn Cu-Fe-Au deposit |
Wang et al.(2011, 2015)按照矿物的期次阶段和空间分布规律,分析了铜陵矿集区新桥矽卡岩铜硫铁矿床、冬瓜山矽卡岩铜金矿床、凤凰山矽卡岩铜铁金矿床磁铁矿、黄铜矿、黄铁矿、矿石、矽卡岩、围岩的Fe同位素组成和变化规律,分析结果表明:形成最早的磁铁矿具有相对较重的δ56Fe值,在此之后形成的黄铁矿,从早到晚,δ56Fe值逐渐变重,这三期中的第一期黄铁矿相对最早期磁铁矿富集Fe的轻同位素(图 3a, c, e),从外矽卡岩到矿体再到内矽卡岩,黄铁矿的δ56Fe值呈现逐渐变轻的趋势(图 3b, d, f);根据以上Fe同位素在时间和空间上的演化特征,结合Fe同位素的分馏机制,反演了流体在时间和空间上的演化过程,进而推断成矿过程:成矿流体在空间上沿着从内矽卡岩-矿体-外矽卡岩的方向演化,先后形成了磁铁矿、第一期胶粒状黄铁矿、第二期细粒黄铁矿、第三期粗粒黄铁矿和黄铜矿;同时,根据矿物Fe同位素与围岩Fe同位素值的对比约束了成矿物质来源,认为Fe来源于岩浆岩而不是围岩;并由此推断:在流体演化过程中,成矿热液相对于成矿有关的岩浆岩富集Fe的轻同位素。相比之下,Wawryk and Foden (2015)在研究澳大利亚Renison钨锡矿床时发现了不同的Fe同位素分馏规律,结果显示黄铁矿(0.61‰~1.14‰)、黄铜矿(0.18‰~0.71‰)、磁铁矿(0.50‰~0.70‰)比矿区花岗岩(0.27‰~0.45‰)具有更重的Fe同位素(图 2),据此提出假设:从具有较重Fe同位素的还原型岩浆演化而来的岩浆热液可以沉淀出具有较重Fe同位素值的矿物,而氧化型岩浆结晶出岩浆型磁铁矿并衍生出具有较轻Fe同位素组成的熔体和流体。
2.1.2 层状镁铁质侵入体、斑岩型铜矿床、矽卡岩矿床——Cu同位素目前为止,很少有实验研究限定Cu同位素分馏机制的相关文献报道,这使得对于高温成矿系统和岩浆系统Cu同位素分馏的解释受到了一定程度的影响和限制。因此,大多数论文试图通过比较Cu同位素值与其他浓度和同位素数据的一致性,对Cu同位素分馏机理进行实证约束。事实上,为了进一步约束Cu同位素可能的指示意义,这些研究都是在已知地球化学反应模型的系统中进行的。尽管如此,这些Cu同位素数据在约束金属来源以及指示找矿勘查等重要方面仍展现出巨大的应用潜力。
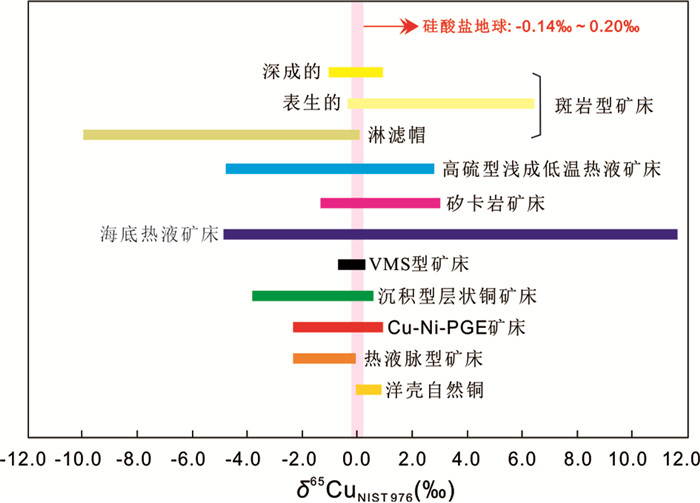
层状镁铁质侵入体和相关的镁铁质型矿床是与硫化物成矿有关的最高温度成矿体系(图 4)。在这些高温成矿体系的成矿过程中,有多个阶段可以导致Cu同位素分馏。Ikehata and Hirata (2012)的研究显示:在硫化物含量极低的基性岩中,单质铜的Cu同位素几乎没有分馏,这表明在没有硫化物分离的情况下,Cu同位素不会发生分馏。相比之下,另外三篇有关Ni-Cu PGE矿床的文献(Malitch et al., 2014; Ripley et al., 2015; Zhao et al., 2017)分别报道了来自俄罗斯诺里尔斯克、美国中部以及中国新疆的相关数据,这些高温成矿体系具有明显的Cu同位素分馏。Malitch et al. (2014)的研究显示从近端的Noril'sk-1侵入体(-0.1‰~0.6‰)到远端的Kharaelakh矿床(-2.3‰~-0.9‰)和Talnakh矿化点(-1.1‰~0.0‰),Cu同位素值有变轻的趋势,作者认为诺里尔斯克地区不同矿床的Cu同位素组成的差异是由于岩浆分异过程中Cu同位素分馏机制的不同、原生母岩浆中Cu同位素比值的差异、或者与外部Cu源发生同化混染作用造成的。Ripley et al. (2015)记录了浸染片状与导管状硫化物矿化的显著差异,并分析了周围沉积岩的Cu同位素组成以评估同化过程中获得铜的可能性,结果表明这两个储库的Cu位素没有明显的对应关系且演化过程也无法精确地模拟,这使得作者能够将分馏机制限制在不同来源的地幔岩浆或岩浆作用过程(如硫化物液体分馏)引起的变化上。Zhao et al. (2017)着重于勘探角度,发现远端体系比近端样品具有更轻的Cu同位素组成,认为Cu同位素的变化是岩浆分异过程中铜进入冷却的基性侵入体不同相时发生的氧化还原反应造成的(图 5)。
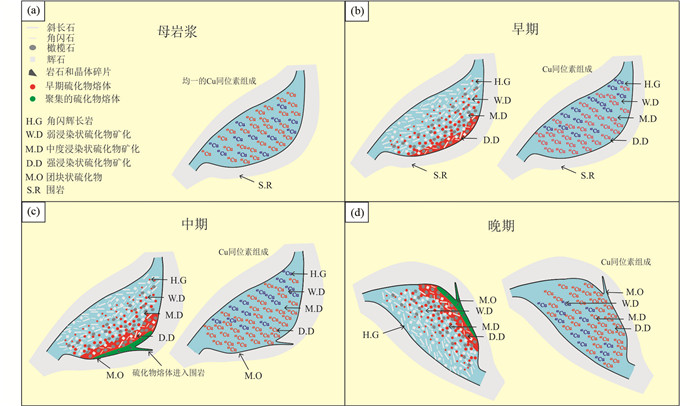
|
图 5 新疆图拉尔根块状硫化物矿床Cu同位素演化和构造演化模式图(据Zhao et al., 2017) Fig. 5 The Cu isotopic evolution and structural evolution of the Tulgren massive sulfide deposit in Xinjiang (after Zhao et al., 2017) |
斑岩型铜矿床是Cu同位素研究中最受关注的矿床类型。Zhu et al. (2000)最先测试并报道了博物馆中相关黄铜矿样品的Cu同位素分馏。随后,Larson et al. (2003)更系统地分析了美国Bagdad和其他斑岩型铜矿床样品的Cu同位素组成,该研究指出高温成矿系统存在明显的Cu同位素变化,并认为这种Cu同位素的系统变化可能与热液体系的物理化学性质有关。Graham et al. (2004)首次采用激光剥蚀法对同一矿床内不同位置的含铜样品进行了详细的Cu同位素分析,研究发现Grasberg矿床样品的Cu同位素组成有着连续变化的特征,晚期侵入体具有更重的Cu同位素组成;而矿床中矽卡岩和周围黄铁矿壳的Cu同位素变化表明这些后续的成矿事件具有不同的地壳金属来源。Li et al. (2010)以热液系统中心区域的黄铜矿为重点研究对象对NorthParkes斑岩型铜矿床进行了研究,结果显示热液中心的黄铜矿具有较轻的Cu同位素组成,由中心向远端δ65Cu值逐渐变重。Dendas (2011)、Mathur et al. (2013)和Song et al. (2016)分别对Bingham、Pebble和德兴斑岩型铜矿床从钾化带到绢英岩化带进行了更大尺度的研究,发现黄铜矿和斑铜矿都有类似的系统变化模式(图 6)。此外,Duan et al. (2016a)在研究西藏地区铁格隆南矿床过程中也发现了类似的Cu同位素变化模式,并根据成矿系统边缘缺乏具有较重δ65Cu值样品的现象,判断整个成矿系统存在部分缺失。综合上述研究结果,勘探地质学家在找矿勘查过程中可以从两个方面对Cu同位素数据加以利用。首先,由于热液成矿系统中由近端到远端Cu同位素组成有系统变化的趋势,因此钻孔中硫化物的Cu同位素组成可以指示成矿流体演化和矿体延伸方向。其次,以上所有研究均表明位于成矿系统中心的含铜样品具有最低的Cu同位素组成。因此,在成矿系统最深处样品的δ65Cu值可能代表了矿床金属来源的Cu同位素组成,或者当这些岩浆系统流体出溶的过程中会发生系统的Cu同位素分馏。因为未蚀变的地幔岩石和硅酸盐地球的δ65Cu值均在0‰附近(Sossi et al., 2015),且大多数斑岩型铜矿床具有相似性,所以这些较低的Cu同位素组成可能代表了这些斑岩型铜矿床的地幔金属来源。随着有关数据的不断积累,混合模型可以用来指示斑岩型铜矿床中幔源金属和壳源金属的不同占比,这可能与矿床的规模有关。
|
图 6 矽卡岩和斑岩型矿床黄铜矿Cu同位素值变化模式图(据Dendas, 2011; Mathur et al., 2013; Song et al., 2016) Fig. 6 Patterns of copper isotope values in chalcopyrite surrounding porphyry copper deposit and skarn deposits (after Dendas, 2011; Mathur et al., 2013; Song et al., 2016) |
相比之下,与斑岩型铜矿床形成相关的其他类型矿化作用还没有得到足够的关注。Maher and Larson (2007)在矽卡岩矿床中发现了与上述斑岩型矿化中黄铜矿相反的Cu同位素系统变化规律,即成矿系统远端样品具有更轻的Cu同位素组成(图 6)。随后,Maher et al. (2011)利用相关实验研究证明热液成矿体系中气液相分离可能引起Cu同位素分馏。Yao et al. (2016)通过对大湖塘矽卡岩矿床进行流体包裹体和Cu-Mo同位素研究,进一步证实了气液相变可以引起金属稳定同位素分馏(Cu-Mo)。在与斑岩型成矿系统有关或者无关的浅成低温热液成矿系统中,自然金和黄铜矿均具有较轻的Cu同位素组成,Saunders et al. (2016)将这一现象归因于纳米级金属颗粒从地幔到浅层地壳的运移。Markl et al. (2006a)研究了大型热液脉成矿系统中含有多种金属的长英质岩中热液脉型黄铜矿的Cu同位素组成,在这种情况下,Cu同位素没有表现出系统性的变化,黄铜矿δ65Cu值集中在0‰左右,并且略呈负值。
2.1.3 VHMS(VMS)型、Irish型矿床——Cu-Zn同位素研究表明VMS型矿床在高温成矿系统中具有较小的Cu同位素变化范围(图 4)。Rouxel et al. (2004)测试了大西洋中脊海底处于活动期VMS矿床相关样品的Cu同位素组成,结果表明δ65Cu值变化范围为3‰,Cu同位素的变化被归因于对海底黑烟囱处早期沉淀硫化物的蚀变重溶和氧化作用。Mason et al. (2005)对俄罗斯乌拉尔山脉泥盆纪的VHMS矿床样品进行了Cu同位素分析,结果显示Cu同位素分馏很小(Δ65Cu≈0.5‰, n=27)。Berkenbosch et al. (2015)则研究了形成于南太平洋的VMS矿床样品,其δ65Cu值变化范围为1.5‰,由于成矿系统中气相被认为具有较高的金属含量,因此作者推断成矿系统的气液相分离是导致该矿床Cu同位素变化的主要原因;对比之下,较早形成且没有处于活动期的古系统中Cu同位素则分馏较小。Ikehata et al. (2011)利用LA-MC-ICP-MS对日本两个Beshi型VMS矿床的黄铜矿、次生孔雀石和自然铜进行了Cu同位素分析,结果显示两个矿床的黄铜矿均具有较小的δ65Cu值变化范围(-0.34‰~0.29‰),但是略有差异,这表明虽然在变质再平衡过程中Cu同位素变化范围有所减小,变质前的Cu同位素特征却基本被保留,并且推断其中一个矿床黄铜矿的Cu同位素组成可能在海底沉淀后蚀变重溶的过程中受到了一定影响。此外,次生孔雀石(2.63‰~2.97‰)和自然铜(1.434‰~1.71‰)均具有比黄铜矿更重的δ65Cu值,这种现象最合理的解释为在低温表生过程中发生的氧化还原反应导致Cu同位素分馏,较重的65Cu优先在Cu(Ⅱ)次生流体中富集,且在Cu(Ⅱ)次生流体中Cu2+还原成自然铜的过程中,自然铜优先富集Cu的轻同位素。
前人研究(Maréchal and Albarède, 2002; John et al., 2008)曾提出一个瑞利模型来解释热液成矿体系中随着时间推移δ66Zn值逐渐变重的现象,具体如下:早期沉淀出的硫化物优先富集Zn的轻同位素,随着硫化物的沉淀,剩余热液流体的Zn同位素组成逐渐变重,导致后期沉淀出的硫化物具有越来越重的δ66Zn值。Fernandez and Borrok (2009)进行连续批式实验研究,结果表明在固溶反应过程中,流体优先富集较重的Zn同位素,为上述瑞利模型提供了实验证据。在矿床学实例研究中,从俄罗斯Alexandrinka VHMS矿床的核部到边部(Mason et al., 2005; 图 7a)以及从爱尔兰Midlands Irish型矿床早阶段到晚阶段(Wilkinson et al., 2005; Gagnevin et al., 2012; 图 7b),闪锌矿的δ66Zn值均展现出逐渐变重的变化趋势,为该瑞利模型提供了实例证据。以上研究表明Zn同位素具有指示成矿流体演化、反演成矿过程、指导找矿勘查的潜力。
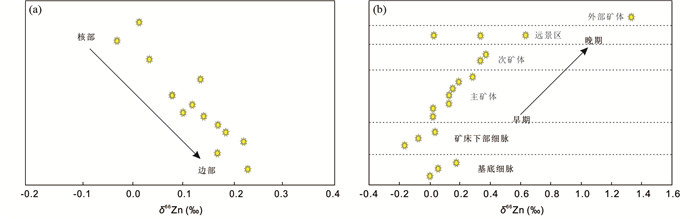
|
图 7 俄罗斯Alexandrinka VHMS矿床闪锌矿的δ66Zn值从核部到边部逐渐变重(a, 据Mason et al., 2005修改)和爱尔兰Midlands Irish型矿床闪锌矿的δ66Zn值从早期到晚期逐渐变重(b, 据Wilkinson et al., 2005修改) Fig. 7 The δ66Zn values gradually increase from core to rim in the Alexandrinka VHMS type deposit in Russia (a, modified after Mason et al., 2005) and from early to late stages in the Midlands Irish-type deposit in Ireland (b, modified after Wilkinson et al., 2005) |
在中低温成矿系统中,由于温度的降低,Fe-Cu-Zn同位素往往展现出更大的变化范围。然而,由于中低温成矿过程中,成矿热液演化较高温成矿过程更为复杂,影响金属稳定同位素分馏的因素更多,因此相关研究较高温成矿系统要薄弱。目前,Fe同位素主要应用于SEDEX矿床和BIF型铁矿的研究中,Cu同位素主要应用于沉积型铜矿床,而Zn同位素主要应用于SEDEX型和MVT型矿床。Fe-Cu是变价元素,即使在中低温成矿系统中,Fe-Cu同位素仍然表现出对氧化还原状态变化的高度敏感性;而Zn在热液中仅以+2价形式存在,不涉及到价态变化,对相态变化引起的瑞利分馏具有很好的约束作用,同时,Zn同位素具有示踪细菌活动对金属成矿贡献的潜力,因为Zn是生物敏感元素,其同位素在有生物活动参与的过程中会产生巨大分馏。因此,在中低温成矿系统中,Fe-Cu-Zn同位素在反演成矿热液的时空演化模式、还原矿床形成过程、示踪成矿物质来源、指示矿体延伸方向、约束矿床成因以及为找矿勘查提供理论依据等方面同样展现出良好的应用前景。
2.2.1 BIF型铁矿床——Fe同位素BIF型铁矿床是一种非常重要的铁矿床类型,前人对世界范围内太古代和元古代BIF型铁矿床进行了Fe同位素研究(李志红等, 2008a, b; 李志红和朱祥坤, 2012; 闫斌等, 2010; Johnson et al., 2003, 2008; Dauphas et al., 2004, 2007; Rouxel et al., 2005; Dauphas and Rouxel, 2006; Johnson and Beard, 2006; Anbar and Rouxel, 2007; Frost et al., 2007; Whitehouse and Fedo, 2007; Steinhoefel et al., 2009, 2010; Planavsky et al., 2009, 2012; Czaja et al., 2010; Tsiko et al., 2010; Heimann et al., 2010; Craddock and Dauphas, 2011; Halverson et al., 2011; Hou et al., 2014),结果表明:世界范围内的BIF型铁矿床具有相似的Fe同位素组成,δ56Fe值变化范围为-2.05‰~3.15‰,最重和最轻的δ56Fe值分别出现在磁铁矿和菱铁矿样品中;相比于其他类型矿床,BIF型铁矿床具有最大的Fe同位素变化范围和相对更重的Fe同位素组成(图 2)。朱祥坤等(2008)认为在BIF型铁矿成矿过程中,随着磁铁矿和赤铁矿的沉淀,成矿热液中剩余Fe2+离子的δ56Fe值越来越轻,随后这些Fe2+离子与CO32-和S2-阴离子结合形成硫化物和碳酸盐矿物。这一假设也得到了其他学者相关研究的证实(Yamaguchi et al., 2005; Archer and Vance, 2006; Severmann et al., 2006; Fehr et al., 2008; Von Blanckenburg et al., 2008; Hofmann et al., 2009; Bekker et al., 2010),研究结果显示沉积型黄铁矿(尤其是2.3Ga前的)和前寒武纪BIF型铁矿床中的菱铁矿均具有相对较轻的Fe同位素组成,δ56Fe值变化范围分别为-3.60‰~1.17‰和-2.04‰~1.04‰。Hou et al. (2014)分析测试山西吕梁BIF型铁矿床中磁铁矿、赤铁矿、菱铁矿等含铁矿物的δ56Fe值:-1.4‰~2.5‰,该变化范围区别于岩浆源Fe同位素(0.1‰)和热液源Fe同位素(-0.5‰)组成,结合矿石主微量元素分析、稀土元素分析、石英的Si-O同位素分析,最终确定该矿床的海相沉积成因。
2.2.2 沉积型铜矿床——Cu同位素Asael et al. (2007)首次对以色列境内Timna沉积型铜矿床开展了Cu同位素研究,并指出Cu同位素在示踪氧化还原反应和约束成矿物质迁移方面具有很好的应用潜力。后来,该研究组通过对德国Kuperschiefer沉积型铜矿床进行扩展研究,厘清了成矿热液中不同离子在相变过程中的化学反应模型(Asael et al., 2009, 2012),进一步证明了Cu同位素可以用来示踪沉积型铜矿床成矿物质来源以及反演中低温成矿过程(100~200℃)中的氧化还原反应。另外,部分学者对非洲的沉积型矿床也进行了Cu同位素研究。Haest et al. (2009)对非洲Dikulushi沉积型铜银矿床进行了详细研究,该矿床经历了多期氧化还原事件且在成矿后又受到表生作用的改造,研究发现辉铜矿的Cu同位素具有系统变化趋势——越靠近成矿系统近端辉铜矿δ65Cu值越重,作者利用这些Cu同位素数据来指示成矿事件或者表生改造过程中较高温度下还原性成矿热卤水的变化。Wilson et al. (2016)测试了非洲赞比亚Trident沉积型铜矿床黄铜矿和自然铜的Cu同位素组成,δ65Cu值变化范围大于6‰。
截至目前为止,关于在沉积型铜矿床的研究中利用Cu同位素示踪金属来源、约束流体运移以及反演成矿过程的相关文献报道仍十分有限,但是鉴于与中低温变质作用有关的成矿过程中,氧化还原条件变化多样,因此关于该类型矿床的Cu同位素应用仍值得进一步深入研究。
2.2.3 SEDEX型矿床-Zn-Fe同位素在2.1.3中我们讨论过,先前研究表明在同一热液体系中,沉淀出的硫化物的δ66Zn值在时间尺度上有逐渐变重的系统变化趋势。基于此,根据内蒙古东升庙SEDEX矿床中主矿体从南西到北东向的横截面上硫化物δ66Zn和δ56Fe值逐渐变重且Pb同位素组成均一的特点,Gao et al. (2018)约束了热液体系的演化过程。类似地,Kelley et al. (2009)对美国阿拉斯加红狗矿区开展Zn同位素研究,发现硫化物Zn同位素组成均一且在时间和空间尺度上均有逐渐变重的系统变化趋势(图 8),并据此反演了成矿热液的时空演化模式。一般而言,在SEDEX型矿床中,时间尺度上逐渐变重的δ66Zn值往往对应着逐渐降低的Fe/Mn比值和Cu浓度(John et al., 2008),这是由于热液混合冷却的过程中Fe和Cu的快速沉淀以及Mn的稳定性造成的(Seewald and Seyfried, 1990; Metz and Trefry, 2000),然而在红狗矿区情况却不同,由于较低的成矿流体温度(115~180℃: Leach et al., 2004; 173~180℃: Stock, 2007)以及成矿体系中的Cu主要以流体包裹体形式而非类质同象替换形式赋存于闪锌矿中,导致硫化物Cu含量较低,因此只有Fe/Mn比值与δ66Zn值展现出负相关关系。根据闪锌矿δ66Zn值(-0.28‰~0.32‰)和δ34S值(-22‰~8‰),结合与元素含量变化的关系,作者判断红狗矿床存在热液型成矿作用,与经典的SEDEX型成矿作用不同。
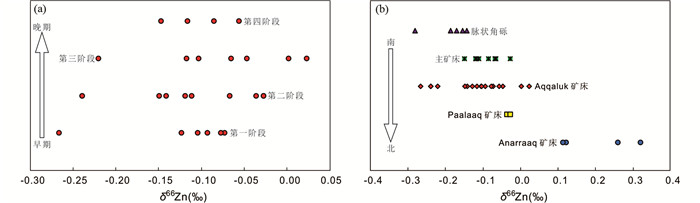
|
图 8 美国阿拉斯加红狗矿区闪锌矿δ66Zn值随时间(a)和空间(b)变化趋势图(据Kelley et al., 2009修改) Fig. 8 The δ66Zn values gradually increase from early to late stages (a) and from south to north (b) in the Red Dog ore district in Alaska (modified after Kelley et al., 2009) |
Wang et al.(2017b, 2018)对西藏北喜马拉雅成矿带扎西康锑铅锌银矿床的黄铁矿、毒砂、闪锌矿、锰铁碳酸盐、围岩(炭质板岩)等展开了Fe-Zn同位素研究,该矿床是北喜马拉雅成矿带唯一的超大型矿床,但成因存在很大争议,主要包括热泉成因(孟祥金等, 2008)、地热卤水成因(张建芳等, 2010)、喷流沉积-叠加改造成因(郑有业等, 2012)、岩浆热液成因(Duan et al., 2016b; Xie et al., 2017; Zhou et al., 2018)。研究结果表明,随着样品蚀变程度的增大,硫化物δ66Zn值逐渐变轻、δ56Fe值逐渐变重(图 9),并利用此相关性成功约束了扎西康矿床的两期叠加成矿作用。同时,Wang et al.(2017b, 2018)建立了扎西康矿床Fe-Zn同位素瑞利分馏模型,并以扎西康矿床实际地质事实为限定条件,计算出扎西康矿床第一期成矿热液的δ56Fe和δ66Zn值变化范围分别为-0.5‰~-1‰和-0.28‰~0‰, 岩浆热液、地热卤水、MVT等成因均无法满足这一Fe-Zn同位素值,只有海底热液系统能产生满足条件的Fe-Zn同位素值(图 2、图 10)。因此,扎西康矿床第一期成矿作用(Pb-Zn)极有可能具有SEDEX成因,至少可以肯定其具有海相成因。
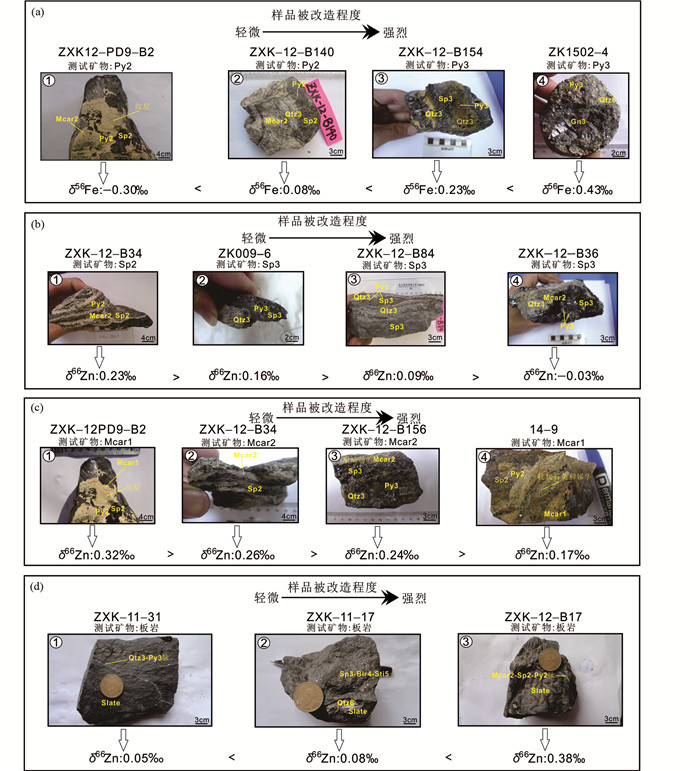
|
图 9 西藏扎西康矿床样品改造程度与Fe-Zn同位素组成之间的关系(据Wang et al., 2017b修改) 样品被改造程度越高:(a)黄铁矿具有越重的δ56Fe值;(b)闪锌矿具有越轻的δ66Zn值;(c)锰铁碳酸盐具有越轻的δ66Zn值;(d)板岩具有越轻的δ66Zn值.矿物缩写:Mcar1-第一阶段锰铁碳酸盐;Mcar2-第二阶段锰铁碳酸盐;Py2-第二阶段黄铁矿;Sp2-第二阶段闪锌矿;Py3-第三阶段黄铁矿;Sp3-第三阶段闪锌矿;Gn3-第三阶段方铅矿;Qtz3-第三阶段石英;Blr4-第四阶段硫锑铅矿;Qtz4-第四阶段石英;Sti5-第五阶段辉锑矿;Qtz6-第六阶段石英 Fig. 9 The relationship between ore alteration and Fe-Zn isotopic compositions in Zhaxikang deposit within Tibet (modified after Wang et al., 2017b) Relationship between increasing alteration of samples and heavier δ56Fe values of pyrite (a), and lighter δ66Zn values of sphalerite (b), and lighter δ66Zn values of Mn-Fe carbonate (c), and lighter δ66Zn values of slate (d). Abbreviations are as follows: Mcar1-stage 1 fine-grained Mn-Fe carbonate; Mcar2-stage 2 coarse-grained Mn-Fe carbonate; Py2-stage 2 pyrite; Sp2-stage 2 sphalerite; Py3-stage 3 pyrite; Sp3-stage 3 sphalerite; Gn3-stage 3 galena; Qtz3-stage 3 quartz; Blr4-stage 4 boulangerite; Qtz4-stage 4 quartz; Sti5-stage 5 stibnite; Qtz6-stage 6 quartz |

|
图 10 不同类型矿床和地质体Zn同位素组成对比图 硅酸盐地球(Chen et al., 2013)、沉积岩(Maréchal et al., 2000; Weiss et al., 2007; Bentahila et al., 2008)、岩浆岩(Viers et al., 2007; Bentahila et al., 2008; Toutain et al., 2008; Herzog et al., 2009; Telus et al., 2012; Chen et al., 2013)、深海碳酸盐(Pichat et al., 2003)、海底热液(John et al., 2008)、深海海水(John and Conway, 2014; Little et al., 2014; Zhao et al., 2014),以及不同成因的矿床:意大利Gorno和Raibl岩浆型铅锌矿床(Maréchal et al., 1999)、中国铜陵矿集区矽卡岩矿床(王跃和朱祥坤, 2010a)、中国西南地区碳酸盐岩容矿型铅锌矿床(Zhou et al., 2014)、爱尔兰Irish型铅锌矿床(Wilkinson et al., 2005)、法国Cévennes MVT型铅锌矿床(Albarède, 2004)、美国阿拉斯加地区红狗SEDEX型铅锌矿床(Kelley et al., 2009)、俄罗斯乌拉尔Alexandrinka VHMS型铅锌矿床(Mason et al., 2005)、中国内蒙古东升庙SEDEX型铅锌矿床(Gao et al., 2018)、中国西藏扎西康锑铅锌银矿床(Wang et al., 2017b, 2018) Fig. 10 Zn isotopic compositions of the different type of deposits and geological mass Bulk Silicate Earth (Chen et al., 2013), sedimentary rocks (Maréchal et al., 2000; Weiss et al., 2007; Bentahila et al., 2008), igneous rocks (Veirs et al., 2007; Bentahila et al., 2008; Toutain et al., 2008; Herzog et al., 2009; Telus et al., 2012; Chen et al., 2013), deep-sea carbonates (Pichat et al., 2003), seafloor hydrothermal fluids (John et al., 2008), deep sea water (John and Conway, 2014; Little et al., 2014; Zhao et al., 2014) and other deposits with different geneses: the Gorno and Raibl magmatic-type deposit in Italy (Maréchal et al., 1999), the skarn-type deposits in the Tongling ore district in China (Wang and Zhu, 2010), the Tianqiao and Bangbangqiao carbonated-hosted Pb-Zn sulphide deposits in China (Zhou et al., 2014), the Irish-type deposit in Ireland (Wilkinson et al., 2005), the Cévennes MVT deposit in France (Albarède, 2004), the Alexandrinka VHMS-type deposit in Russia (Mason et al., 2005), the Red Dog SEDEX-type ore district in Alaska (Kelley et al., 2009), Dongshengmiao SEDEX-type deposit in China (Gao et al., 2017) and the Zhaxikang Sb-Pb-Zn-Ag deposit in China (Wang et al., 2017b, 2018) |
在过去的几十年,细菌与矿床之间的关系,尤其是细菌在低温矿床成矿作用中所扮演的角色受到了广泛关注。锌(Zn)和镉(Cd)不仅是闪锌矿的直接矿化金属,也是生物敏感元素,它们的同位素在有生物活动参与的过程中会产生巨大分馏,这使得Zn-Cd同位素具有示踪细菌活动对金属成矿贡献的潜力。Li et al. (2019)对金顶MVT超大型铅锌矿床中原生闪锌矿进行了Zn-Cd-S同位素研究,取得了以下创新性成果和认识:(1)在镜下发现了具有细菌成因结构的硫化物,闪锌矿具有极低的S同位素组成,表明了成矿所需的还原性硫来自于细菌还原硫酸盐过程。细菌还原硫酸盐过程的S同位素组成变化满足瑞利分馏模型,受控于硫酸盐的还原程度。换言之,闪锌矿的S同位素组成越重说明其硫酸盐还原程度愈高,细菌活动性愈强;(2)与目前已报道的其他矿床相比,金顶闪锌矿具有最轻的Zn和Cd同位素组成。其Zn同位素组成与Zn/Cd比值存在很好的正相关关系,说明该热液系统早期沉淀的闪锌矿就已经具有极轻的Zn同位素组成。金顶矿区存在大量有机质,细菌还原硫酸盐的同时,会将复杂有机质降解成可溶的有机羧酸,这些可溶的有机羧酸具有极强络合金属的能力,并且它们会优先络合重的66Zn和114Cd。Zn同位素组成与S同位素组成存在负相关关系,这说明早期沉淀的闪锌矿极轻的Zn同位素组成是由于含Zn成矿流体中大量的自由Zn被强的细菌活动性所产生的可溶有机羧酸络合;(3)模拟计算表明要产生如此轻的Zn同位素值,需要成矿流体中90%的Zn与细菌新陈代谢产生的可溶有机羧酸络合。这指示细菌活动所产生的可溶有机羧酸与Zn的络合是金顶铅锌矿运移金属Zn的重要机制。
2.2.5 碳酸盐岩容矿型铅锌矿床——Zn同位素Zhou et al. (2014)开展了中国西南地区(四川-云南-贵州铅锌矿集区)碳酸盐岩容矿型铅锌矿床的Zn同位素研究,根据Zn同位素从早期到晚期逐渐富集Zn重同位素的特征,结合流体包裹体测温的数据(120~260℃),确定瑞利分馏是造成Zn同位素组成变化的主要原因,反演了成矿过程。同时,与世界范围内不同成因铅锌矿床闪锌矿Zn同位素组成对比(图 10),包括美国阿拉斯加地区红狗SEDEX型铅锌矿床(-0.28‰~0.32‰; Kelley et al., 2009)、俄罗斯乌拉尔Alexandrinka VHMS型铅锌矿床(-0.48‰~-0.05‰; Mason et al., 2005)、爱尔兰Irish型铅锌矿床(-0.45‰~1.05‰; Wikinson et al., 2005)、法国Cévennes MVT型铅锌矿床(-0.34‰~0.19‰; Albarède, 2004)以及意大利Gorno和Raibl岩浆型铅锌矿床(-0.26‰~0.16‰; Maréchal et al., 1999),结合S-Pb同位素分析,确定了碳酸盐岩容矿型铅锌矿床的特殊成因,推断这些矿床成矿物质来源于古生代碳酸盐和前寒武纪基底,而这些与成矿有关的岩石(-0.52‰~0.16‰)具有比闪锌矿(天桥:-0.54‰~0.30‰;板板桥:-0.21‰~0.43‰)轻的Zn同位素组成,这与前人实验和实际研究结果相吻合(Maréchal and Albarède, 2002; John et al., 2008; Fernandez and Borrok, 2009):相对于流体,固体优先富集Zn的轻同位素。
2.3 表生成矿系统随着温度的进一步降低,在表生成矿系统中,金属稳定同位素通常具有最大的变化范围,这为找矿勘查提供了一个很好的切入点,我们可以利用地表表生成矿系统中矿石、风化的岩石、土壤、水、植物等展现出的较大同位素分馏作为矿产勘查的指示标志。目前,暂无Zn同位素应用于表生成矿系统的实例研究。由于表生成矿系统中,往往具有丰富的含铁和含铜矿物,因此Fe-Cu同位素应用比较广泛。Fe同位素主要用于追踪表生地球化学循环和矿床形成/演化过程;Cu同位素则更多地被用来尝试指示隐伏矿体。
2.3.1 铁矿床——Fe同位素Markl et al. (2006b)测试了德国Schwarzward热液脉型矿床中黄铁矿、赤铁矿、针铁矿、菱铁矿、含铁砷酸盐矿物和沉积型铁矿石的Fe同位素组成,结果显示高温蚀变会使矿物δ56Fe值变重,而低温蚀变几乎不改变矿物的Fe同位素组成。类似地,Cheng et al. (2015)研究了中国云南高松锡多金属矿床的Fe同位素组成和变化(-0.33‰~0.20‰),结果显示δ56Fe值和氧化程度具有正相关关系,具体如下:原生硫化物矿石的δ56Fe值比氧化矿石低,而氧化程度最高的铁帽样品具有最重的Fe同位素组成(图 11)。以上研究结果表明,在高松矿床表生风化过程中,Fe同位素发生了明显分馏,且氧化程度越高的样品优先富集Fe的重同位素。此外,在高松矿区,从岩浆岩到地表,δ56Fe值具有逐渐变重的空间变化趋势,这证实了该矿床的金属来源于岩浆岩而非围岩。同时,原生硫化物富集Fe的轻同位素说明母岩浆流体出溶的过程中也有Fe同位素的分馏,且矽卡岩矿床的铁具有岩浆来源。这两项研究证明Fe同位素具有追踪表生地球化学循环和矿床形成/演化过程的潜力。
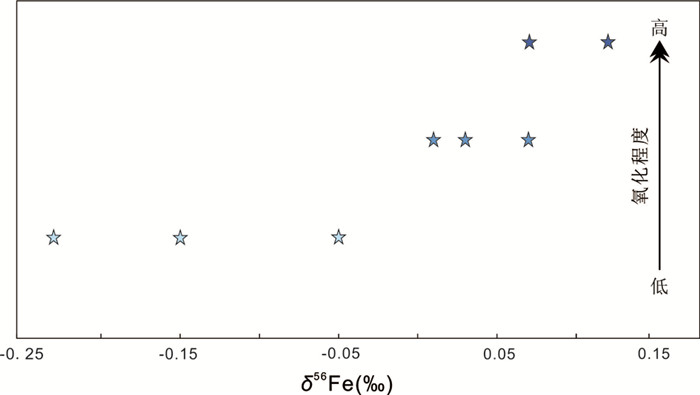
|
图 11 中国云南高松锡多金属矿床的Fe同位素组成与矿石氧化程度呈正相关关系(据Cheng et al., 2015修改) Fig. 11 Fe isotopic compositions of the oxidized ores with various oxidation degrees from the Gaosong deposit, China (modified after Cheng et al., 2015) |
前人分别从理论实验和野外矿床实践两方面对表生成矿系统开展Cu同位素相关研究,结果显示表生成矿系统具有最大的Cu同位素变化范围,δ65Cu值变化可达20‰。在本节中,我们将首先聚焦于怎样利用实验研究约束Cu同位素分馏机制,然后展示如何将自然界中岩石、土壤、水和植物中发现的Cu同位素分馏利用于矿床学研究以及找矿勘查。
多项研究已经清楚地表明,在涉及铜的不同反应中,电子迁移导致的Cu同位素分馏最大可达3‰,氧化态通常富集重的Cu同位素,而还原态则富集轻的Cu同位素。例如,Maréchal et al. (1999)在利用MP-1树脂提纯铜的过程中,通过改变键合环境首次展示了这种电子迁移相关的Cu同位素分馏;Zhu et al. (2002)和Ehrlich et al. (2004)对不同的含铜矿物进行氧化还原反应研究,发现δ65Cu值变化为3‰;Mathur et al. (2005)则发现辉铜矿的氧化过程会比黄铜矿的氧化过程在热液中产生稍重的δ65Cu值。Wall et al.(2011a, b)利用实时同步加速器XRD数据研究表生氧化过程中与辉铜矿和斑铜矿氧化有关的Cu同位素分馏,结果显示动力学化学反应会影响流体及不同含铜矿物的Cu同位素组成,且辉铜矿展现出最大的Cu同位素变化范围。因此,作者推断随着表生氧化反应的进行,含铜矿物中Cu的氧化最可能与成矿流体Cu同位素组成变化有关。另一方面,生物作用也可以改变溶液和固体的Cu同位素组成,从微观的微生物到肉眼可见的植物,相关的生物作用都对Cu同位素分馏有着重要的影响(Fujii et al., 2013; Kimball et al., 2009; Navarrete et al., 2011a, b)。尽管不同的实验研究中,Cu同位素分馏程度有所差异,但是生物物质一般优先富集较轻的Cu同位素。然而,与溶液中的铜相比,表生系统中的铜往往与细菌无关。因此,相比于有机物质,无机反应可能是表生系统中铜的主要来源。
实验数据可以应用于自然体系,在表生成矿系统中,淋滤帽、富集层和原生矿石的Cu同位素组成有显著差别,一般而言,淋滤帽比富集层和原生矿具有更低的δ65Cu值,而富集层往往具有最重的δ65Cu值(图 12)。在次生成矿过程中,铜的运移和沉淀在土壤以及淋滤帽铁氧化物中产生的含铜矿物一般具有相对较低的Cu同位素组成,但是δ65Cu值变化范围较大(-9.0‰~-2.0‰; Braxton and Mathur, 2011; Mathur et al., 2010, 2013; Mathur and Schlitt, 2010; Mirnejad et al., 2010)。

|
图 12 表生环境中铜的三个主要储层Cu同位素组成差异明显(据Mathur and Fantle, 2015修改) Fig. 12 Distinct copper isotope compositions in the three main reservoirs in the supergene environment (modified after Mathur and Fantle, 2015) |
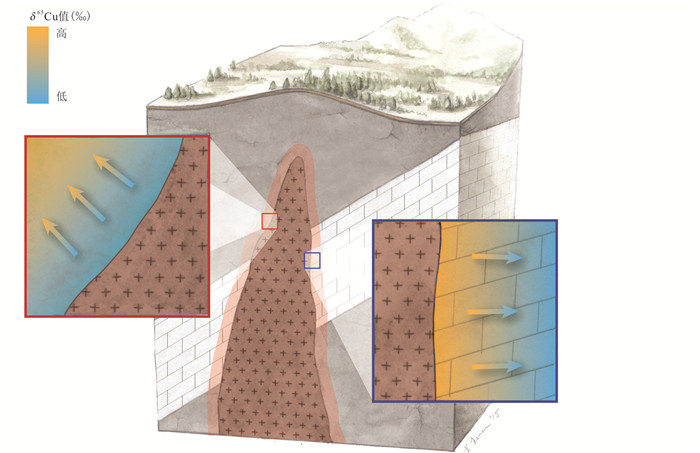
从勘查地球化学的角度来看,在表生成矿系统中Cu同位素数据可以从几个不同方面加以利用。首先就是Mathur et al. (2005)提出的含有较重Cu同位素组成的流体可以用来指示含铜硫化物的表生作用,前人的诸多研究也都记录了这种关系(Borrok et al., 2008; Kimball et al., 2009; Mathur et al., 2013, 2014)。例如,Kimball et al. (2009)的研究发现在一处ADM水系中,Cu同位素组成由近源的较重值过渡到远源的较轻值。Mathur et al. (2013)基于这一想法进行了更大尺度的研究,发现在著名的阿拉斯加Pebble斑岩型铜矿床数十千米范围内,存在具有较重Cu同位素组成的水系,作者认为是含铜矿物的表生氧化作用造成的。随后,Mathur et al. (2014)进一步证明了以上观点,研究显示铜矿床中不同含铜矿物的表生氧化作用将造成对应流体具有不同的Cu同位素组成,而流体的Cu同位素组成与被氧化含铜矿物的矿物学特征密切相关(图 13)。因此,该研究表明水系的Cu同位素特征具有矿物学指示意义,如果硫化物初始δ65Cu值可以确定,那么水系中Cu同位素组成可以用来推断被风化矿物的类型。其次,表生过程中的残余物质,例如土壤和淋滤帽中的铁氧化物,也可以作为表生过程的指示标志并且应用于找矿勘查。Mirnejad et al. (2010)和Braxton and Mathur (2011)的研究显示铁氧化物比富集层辉铜矿和原生矿具有更轻的Cu同位素组成,作者们提出在淋滤帽中,辉铜矿发生表生作用的位置比未发生表生作用的区域具有更重的Cu同位素组成。在以上两个研究中,两个研究区(伊朗和菲律宾)的淋滤帽都具有较轻的Cu同位素组成,但是无法与非矿化区进行对比。Mathur and Schlitt (2010)展示了秘鲁Queaveco矿区的Cu同位素数据,与深部未矿化区域相比,位于深部富集层以上的铁氧化物均具有更重的Cu同位素组成。

|
图 13 溶液与含铜矿物之间的Cu同位素分馏系数来用于鉴别被风化的含铜矿物 阴影区为黄铜矿和辉铜矿的理论δ65Cu值变化范围(据Mathur et al., 2014修改) Fig. 13 Fractionation factors between the solution and the economic copper mineral of interest used to identify copper mineral being weathered Shaded areas indicate the experimental ranges for weathering of chalcopyrite and chalcocite (modified after Mathur et al., 2014) |
通过铜的横向迁移形成的新的表生矿床中,铜的氧化物和硫化物可以用来示踪初始物源。Braxton and Mathur (2011)对铜横向迁移形成的辉铜矿进行了Cu同位素测试,结果显示距离原生斑岩型铜矿床源头最近的辉铜矿具有最重的Cu同位素组成。类似地,Palacios et al. (2011)也记录了Atacama风化矿床中氧化相相关的铜迁移及循环。以上研究表明,在表生地质过程中Cu同位素分馏程度和铜循环程度成正比。Mathur et al. (2010)对美国亚利桑那州斑岩型铜矿床表生样品的相关研究也证明了这一点,该研究指出相对较大的铜循环可能会导致表生成矿作用中铜总量的减少,进而加大表生辉铜矿和初始黄铜矿Cu同位素组成的差异。
另一方面,由于土壤的Cu同位素组成相对比较复杂,故而目前还没有直接将其应用于矿床学研究和找矿勘查的实例。大多数相关研究都倾向于应用在环境监测方面,主要包括矿石和土壤Cu同位素对比对物源的指示以及铜迁移和土壤Cu同位素组成的相关性(Pokrovsky et al., 2008; Bigalke et al., 2010a, b, 2011; Babcsányi et al., 2016; Song et al., 2016)。从一般意义上来说,土壤的Cu同位素组成在一定程度上代表了其母岩的Cu同位素组成。如果土壤母岩的Cu同位素组成高于不含铜硫化物的岩石,则这种土壤Cu同位素组成的差异可以指示深部的矿化。针对此现象,Mathur et al. (2012)研究了淋滤帽中富含黄铁矿的黑色页岩的风化作用,展示了淋滤帽中观察到的Cu同位素分馏现象。从理论上来讲,富含硫化物的母岩风化产生的土壤,其Cu同位素组成可以作为指示深部矿化的标志。
自然水系在流经地下硫化物储层时,会溶解部分硫化物中的元素,溶解了硫化物中铜元素水系的Cu同位素组成与硫化物矿石初始的Cu同位素组成有直接关系。在溶解的过程中,65Cu的析出量要远大于63Cu,这导致流经过硫化物储层的水系Cu同位素组成变重。因此,从找矿勘查的角度,水系的Cu同位素组成也可以指示深部矿化。海水和自然水系的Cu同位素组成要比硅酸盐地球的平均Cu同位素组成更重,约为0.6‰(Bermin et al., 2006; Vance et al., 2008; Little et al., 2014),这可能就与硫化物的风化作用有关(Mathur and Fantle, 2015)。以下两项研究作为应用实例,证实了水系Cu同位素测定在找矿勘查中的应用前景。Mathur et al. (2013)展示了美国阿拉斯加地区Pebble斑岩型铜矿床渗出水的Cu同位素数据,该矿床发生了强烈的表生风化作用,测试的水系样品均采自距矿床垂直距离100m以上的冰碛物。结果显示,直接采自于矿床上方的水系样品具有较重的Cu同位素组成(平均δ65Cu值为1.47‰),而矿化区以外采集的水系样品Cu同位素组成则较轻(平均δ65Cu值为-0.27‰)。同时,元素含量测定结果表明,风化作用过程中,溶解了硫化物的水系的Cu含量仅为5×10-9。在这种情况下,通过Cu同位素组成的差异指示深部矿化比元素异常更加直接有效。在此项研究基础上,Mathur et al. (2014)将研究扩展到拥有不同类型铜矿床的地区,发现较重的δ65Cu值始终出现在发生铜硫化物风化作用的区域。同时,矿床类型与测定的δ65Cu值没有直接关系,而含Cu矿物类型对水系Cu同位素组成有影响。
3 总结与展望本文系统统计和总结了世界范围内相关矿石、水、岩石、土壤和植物等自然物质的Fe-Cu-Zn同位素研究成果。Fe-Zn同位素在岩浆过程、流体出溶、矿物沉淀和表生过程中均发生明显分馏,不同类型的矿床具有不同的Fe-Zn同位素特征和变化历史,因此,Fe-Zn同位素具有示踪金属来源和反演成矿过程的潜力。相比之下,Cu同位素的研究更加全面细致,在不同的矿床中,Cu同位素的应用范围从矿区到非矿化区,跨度从数米到数千米不等,鉴于当前Cu同位素的两种常用测试方法都可以在纳克范围内精确测定Cu同位素组成,且Cu同位素具有多种特殊的地球化学特性,因此,Cu同位素可以为矿床学研究和找矿勘查提供多种有用的信息。
在未来矿床学研究以及找矿勘查中,金属稳定同位素数据可以从三方面加以利用。首先,地表的植物、水、风化的岩石以及土壤中产生的较大的同位素分馏可以作为地下矿产勘查的指示标志;第二,矿区范围内金属稳定同位素往往具有系统的空间变化规律,可以指示成矿热液空间演化模式和矿体延伸方向;第三,金属元素作为成矿元素,其同位素可以直接有效地约束矿石的形成过程、成因以及源区特征。
此外,未来的金属稳定同位素研究还需要重点关注不具备开采价值的成矿系统,以确定它们与工业矿床之间的关系与区别;进一步深入研究如何利用地表风化岩石、土壤、水、植物的金属稳定同位素体系来确定深部矿化;关注不同种类金属稳定同位素在矿床学研究以及找矿勘查中应用时表现出的异同点,展开多种金属稳定同位素联合应用研究。
Albarède F. 2004. The stable isotope geochemistry of copper and zinc. Reviews in Mineralogy and Geochemistry, 55(1): 409-427 DOI:10.2138/gsrmg.55.1.409 |
Anbar AD and Rouxel O. 2007. Metal stable isotopes in paleoceanography. Annual Review of Earth and Planetary Sciences, 35: 717-746 DOI:10.1146/annurev.earth.34.031405.125029 |
Archer C and Vance D. 2006. Coupled Fe and S isotope evidence for Archean microbial Fe(Ⅲ) and sulfate reduction. Geology, 34(3): 153-156 DOI:10.1130/G22067.1 |
Archer C, Andersen MB, Cloquet C, Conway TM, Dong SF, Ellwood M, Moore R, Nelson J, Rehkämper M, Rouxel O, Samanta M, Shin KC, Sohrin Y, Takano S and Wasylenki L. 2017. Inter-calibration of a proposed new primary reference standard AA-ETH Zn for zinc isotopic analysis. Journal of Analytical Atomic Spectrometry, 32(2): 415-419 DOI:10.1039/C6JA00282J |
Asael D, Matthews A, Bar-Matthews M and Halicz L. 2007. Copper isotope fractionation in sedimentary copper mineralization (Timna Valley, Israel). Chemical Geology, 243(3-4): 238-254 DOI:10.1016/j.chemgeo.2007.06.007 |
Asael D, Matthews A, Oszczepalski S, Bar-Matthews M and Halicz L. 2009. Fluid speciation controls of low temperature copper isotope fractionation applied to the Kupferschiefer and Timna ore deposits. Chemical Geology, 262(3-4): 147-158 DOI:10.1016/j.chemgeo.2009.01.015 |
Asael D, Matthews A, Bar-Matthews M, Harlavan Y and Segal I. 2012. Tracking redox controls and sources of sedimentary mineralization using copper and lead isotopes. Chemical Geology, 310-311: 23-35 DOI:10.1016/j.chemgeo.2012.03.021 |
Babcsányi I, Chabaux F, Granet M, Meite F, Payraudeau S, Duplay J and Imfeld G. 2016. Copper in soil fractions and runoff in a vineyard catchment: Insights from copper stable isotopes. Science of The Total Environment, 557-558: 154-162 DOI:10.1016/j.scitotenv.2016.03.037 |
Balistrieri LS, Borrok DM and Wanty RB and Ridle WI. 2008. Fractionation of Cu and Zn isotopes during adsorption onto amorphous Fe(Ⅲ) oxyhydroxide: Experimental mixing of acid rock drainage and ambient river water. Geochimica et Cosmochimica Acta, 72(2): 311-328 DOI:10.1016/j.gca.2007.11.013 |
Beard BL and Johnson CM. 1999. High precision iron isotope measurements of terrestrial and lunar materials. Geochimica et Cosmochimica Acta, 63(11-12): 1653-1660 DOI:10.1016/S0016-7037(99)00089-7 |
Beard BL, Johnson CM, Skulan JL, Nealson KH, Cox L and Sun H. 2003a. Application of Fe isotopes to tracing the geochemical and biological cycling of Fe. Chemical Geology, 195(1-4): 87-117 DOI:10.1016/S0009-2541(02)00390-X |
Beard BL, Johnson CM, Von Damm KL and Poulson RL. 2003b. Iron isotope constraints on Fe cycling and mass balance in oxygenated earth oceans. Geology, 31(7): 629-632 DOI:10.1130/0091-7613(2003)031<0629:IICOFC>2.0.CO;2 |
Bekker A, Slack JF, Planavsky N, Krapeň B, Hofmann A, Konhauser KO and Rouxel OJ. 2010. Iron formation: The sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Economic Geology, 105(3): 467-508 DOI:10.2113/gsecongeo.105.3.467 |
Belshaw NS, Zhu XK, Guo Y and O'Nions RK. 2000. High precision measurement of iron isotopes by plasma source mass spectrometry. International Journal of Mass Spectrometry, 197(1-3): 191-195 DOI:10.1016/S1387-3806(99)00245-6 |
Bennett SA, Rouxel O, Schmidt K, Garbe-Schöznberg D, Statham PJ and German CR. 2009. Iron isotope fractionation in a buoyant hydrothermal plume, 5°S Mid-Atlantic Ridge. Geochimica et Cosmochimica Acta, 73(19): 5619-5634 DOI:10.1016/j.gca.2009.06.027 |
Bentahila Y, Othman DB and Luck JM. 2008. Strontium, lead and zinc isotopes in marine cores as tracers of sedimentary provenance: A case study around Taiwan orogen. Chemical Geology, 248(1-2): 62-82 DOI:10.1016/j.chemgeo.2007.10.024 |
Berkenbosch HA, de Ronde CEJ, Paul BT and Gemmell JB. 2015. Characteristics of Cu isotopes from chalcopyrite-rich black smoker chimneys at Brothers volcano, Kermadec arc, and Niuatahi volcano, Lau basin. Mineralium Deposita, 50(7): 811-824 DOI:10.1007/s00126-014-0571-y |
Bermin J, Vance D, Archer C and Statham PJ. 2006. The determination of the isotopic composition of Cu and Zn in seawater. Chemical Geology, 226(3-4): 280-297 DOI:10.1016/j.chemgeo.2005.09.025 |
Bigalke M, Weyer S and Wilcke W. 2010a. Stable copper isotopes: A novel tool to trace copper behavior in hydromorphic soils. Soil Science Society of America Journal, 74(1): 60-73 DOI:10.2136/sssaj2008.0377 |
Bigalke M, Weyer S, Kobza J and Wilcke W. 2010b. Stable Cu and Zn isotope ratios as tracers of sources and transport of Cu and Zn in contaminated soil. Geochimica et Cosmochimica Acta, 74(23): 6801-6813 DOI:10.1016/j.gca.2010.08.044 |
Bigalke M, Weyer S and Wilcke W. 2011. Stable Cu isotope fractionation in soils during oxic weathering and podzolization. Geochimica et Cosmochimica Acta, 75(11): 3119-3134 DOI:10.1016/j.gca.2011.03.005 |
Bilenker LD, Simon AC, Reich M, Lundstrom CC, Gajos N, Bindeman I, Barra F and Munizaga R. 2016. Fe-O stable isotope pairs elucidate a high-temperature origin of Chilean iron oxide-apatite deposits. Geochimica et Cosmochimica Acta, 177: 94-104 DOI:10.1016/j.gca.2016.01.009 |
Borrok DM, Wanty RB, Ridley WI, Wolf R, Lamothe PJ and Adams M. 2007. Separation of copper, iron, and zinc from complex aqueous solutions for isotopic measurement. Chemical Geology, 242(3-4): 400-414 DOI:10.1016/j.chemgeo.2007.04.004 |
Borrok DM, Nimick DA, Wanty RB and Ridley WI. 2008. Isotopic variations of dissolved copper and zinc in stream waters affected by historical mining. Geochimica et Cosmochimica Acta, 72(2): 329-344 DOI:10.1016/j.gca.2007.11.014 |
Braxton D and Mathur R. 2011. Exploration applications of copper isotopes in the supergene environment: A case study of the bayugo porphyry copper-gold deposit, Southern philippines. Economic Geology, 106(8): 1447-1463 DOI:10.2113/econgeo.106.8.1447 |
Chapman JB, Mason TFD, Weiss DJ, Coles BJ and Wilkinson JJ. 2006. Chemical separation and isotopic variations of Cu and Zn from five geological reference materials. Geostandards and Geoanalytical Research, 30(1): 5-16 DOI:10.1111/j.1751-908X.2006.tb00907.x |
Chen H, Savage PS, Teng FZ, Helz RT and Moynier F. 2013. Zinc isotope fractionation during magmatic differentiation and the isotopic composition of the bulk Earth. Earth and Planetary Science Letters, 369-370: 34-42 DOI:10.1016/j.epsl.2013.02.037 |
Cheng YB, Mao JW, Zhu XK and Wang Y. 2015. Iron isotope fractionation during supergene weathering process and its application to constrain ore genesis in Gaosong deposit, Gejiu district, SW China. Gondwana Research, 27(3): 1283-1291 DOI:10.1016/j.gr.2013.12.006 |
Craddock PR and Dauphas N. 2011. Iron and carbon isotope evidence for microbial iron respiration throughout the Archean. Earth and Planetary Science Letters, 303(1-2): 121-132 DOI:10.1016/j.epsl.2010.12.045 |
Czaja AD, Johnson CM, Beard BL, Eigenbrode JL, Freeman KH and Yamaguchi KE. 2010. Iron and carbon isotope evidence for ecosystem and environmental diversity in the ~2. 7 to 2.5Ga Hamersley Province, Western Australia. Earth and Planetary Science Letters, 292(1-2): 170-180 |
Dauphas N, Janney PE, Mendybaev RA, Wadhwa M, Richter FM, Davis AM, van Zuilen M, Hines R and Foley CN. 2004. Chromatographic separation and multicollection-ICPMS analysis of iron. Investigating mass-dependent and -independent isotope effects. Analytical Chemistry, 76(19): 5855-5863 |
Dauphas N and Rouxel O. 2006. Mass spectrometry and natural variations of iron isotopes. Mass Spectrometry Reviews, 25(4): 515-550 |
Dauphas N, Van Zuilen M, Busigny V, Lepland A, Wadhwa M and Janney PE. 2007. Iron isotope, major and trace element characterization of Early Archean supracrustal rocks from SW Greenland: Protolith identification and metamorphic overprint. Geochimica et Cosmochimica Acta, 71(19): 4745-4770 DOI:10.1016/j.gca.2007.07.019 |
Dauphas N, Teng FZ and Arndt NT. 2010. Magnesium and iron isotopes in 2. 7Ga Alexo komatiites: Mantle signatures, no evidence for Soret diffusion, and identification of diffusive transport in zoned olivine. Geochimica et Cosmochimica Acta, 74(11): 3274-3291 DOI:10.1016/j.gca.2010.02.031 |
Dekov VM, Rouxel O, Asael D, Hålenius U and Munnik F. 2013. Native Cu from the oceanic crust: Isotopic insights into native metal origin. Chemical Geology, 359: 136-149 DOI:10.1016/j.chemgeo.2013.10.001 |
Dendas M. 2011. Copper isotope tracers of fluid flow at Bingham Canyon mine, Utah. Master Degree Thesis. Tuscon: University of Arizona, 1-36
|
Duan JL, Tang JX, Li YB, Liu SA, Wang Q, Yang C and Wang YY. 2016a. Copper isotopic signature of the Tiegelongnan high-sulfidation copper deposit, Tibet: Implications for its origin and mineral exploration. Mineralium Deposita, 51(5): 591-602 DOI:10.1007/s00126-015-0624-x |
Duan JL, Tang JX and Lin B. 2016b. Zinc and lead isotope signatures of the Zhaxikang Pb-Zn deposit, South Tibet: Implications for the source of the ore-forming metals. Ore Geology Reviews, 78: 58-68 DOI:10.1016/j.oregeorev.2016.03.019 |
Ehrlich S, Butler I, Halicz L, Rickard D, Oldroyd A and Matthews A. 2004. Experimental study of the copper isotope fractionation between aqueous Cu(Ⅱ) and covellite, CuS. Chemical Geology, 209(3-4): 259-269 DOI:10.1016/j.chemgeo.2004.06.010 |
Fehr MA, Andersso PS, Hålenius U and Mörth CM. 2008. Iron isotope variations in Holocene sediments of the Gotland Deep, Baltic Sea. Geochimica et Cosmochimica Acta, 72(3): 807-826 DOI:10.1016/j.gca.2007.11.033 |
Fernandez A and Borrok DM. 2009. Fractionation of Cu, Fe, and Zn isotopes during the oxidative weathering of sulfide-rich rocks. Chemical Geology, 264(1-4): 1-12 DOI:10.1016/j.chemgeo.2009.01.024 |
Foden J, Sossi PA and Wawryk CM. 2015. Fe isotopes and the contrasting petrogenesis of A-, I- and S-type granite. Lithos, 212-215: 32-44 DOI:10.1016/j.lithos.2014.10.015 |
Frost CD, Von Blanckenburg F, Schoenberg R, Frost BR and Swapp SM. 2007. Preservation of Fe isotope heterogeneities during diagenesis and metamorphism of banded iron formation. Contributions to Mineralogy and Petrology, 153(2): 211-235 DOI:10.1007/s00410-006-0141-0 |
Fujii T, Moynier F, Abe M, Nemoto K and Albarède F. 2013. Copper isotope fractionation between aqueous compounds relevant to low temperature geochemistry and biology. Geochimica et Cosmochimica Acta, 110: 29-44 DOI:10.1016/j.gca.2013.02.007 |
Gagnevin D, Boyce AJ, Barrie CD, Menug JF and Blakeman RJ. 2012. Zn, Fe and S isotope fractionation in a large hydrothermal system. Geochimica et Cosmochimica Acta, 88: 183-198 DOI:10.1016/j.gca.2012.04.031 |
Gao ZF, Zhu XK, Sun J, Luo ZH, Bao C, Tang C and Ma JX. 2018. Spatial evolution of Zn-Fe-Pb isotopes of sphalerite within a single ore body: A case study from the Dongshengmiao ore deposit, Inner Mongolia, China. Mineralium Deposita, 53(1): 55-65 DOI:10.1007/s00126-017-0724-x |
Graham S, Pearson N, Jackson S, Griffin W and O'Reilly SY. 2004. Tracing Cu and Fe from source to porphyry: In situ determination of Cu and Fe isotope ratios in sulfides from the Grasberg Cu-Au deposit. Chemical Geology, 207(3-4): 147-169 DOI:10.1016/j.chemgeo.2004.02.009 |
Haest M, Muchez P, Petit JCJ and Vanhaecke F. 2009. Cu isotope ratio variations in the Dikulushi Cu-Ag deposit, DRC: Of primary origin or induced by supergene reworking?. Economic Geology, 104(7): 1055-1064 DOI:10.2113/econgeo.104.7.1055 |
Halverson GP, Poitrasson F, Hoffman PF, Nédélec A, Montel JM and Kirby J. 2011. Fe isotope and trace element geochemistry of the Neoproterozoic syn-glacial Rapitan iron formation. Earth and Planetary Science Letters, 309(1-2): 100-112 DOI:10.1016/j.epsl.2011.06.021 |
Heimann A, Beard BL and Johnson CM. 2008. The role of volatile exsolution and sub-solidus fluid/rock interactions in producing high 56Fe/54Fe ratios in siliceous igneous rocks. Geochimica et Cosmochimica Acta, 72(17): 4379-4396 DOI:10.1016/j.gca.2008.06.009 |
Heimann A, Johnson CM, Beard BL, Valley JW, Roden EE, Spicuzza MJ and Beukes NJ. 2010. Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ~2. 5Ga marine environments. Earth and Planetary Science Letters, 294(1-2): 8-18 DOI:10.1016/j.epsl.2010.02.015 |
Herzog GF, Moynier F, Albarède F and Berezhnoy AA. 2009. Isotopic and elemental abundances of copper and zinc in lunar samples, Zagami, Pele's hairs, and a terrestrial basalt. Geochimica et Cosmochimica Acta, 73(19): 5884-5904 DOI:10.1016/j.gca.2009.05.067 |
Hofmann A, Bekker A, Rouxel O, Rumble D and Master S. 2009. Multiple sulphur and iron isotope composition of detrital pyrite in Archaean sedimentary rocks: A new tool for provenance analysis. Earth and Planetary Science Letters, 286(3-4): 436-445 DOI:10.1016/j.epsl.2009.07.008 |
Hou KJ, Li YH, Gao JF, Liu F and Qin Y. 2014. Geochemistry and Si-O-Fe isotope constraints on the origin of banded iron formations of the Yuanjiacun Formation, Lvliang Group, Shanxi, China. Ore Geology Reviews, 57: 288-298 DOI:10.1016/j.oregeorev.2013.09.018 |
Housh T and Çiftçi E. 2008. Cu isotope geochemistry of volcanogenic massive sulphide deposits of the eastern Pontides, Turkey. IOP Conference Series: Earth and Environmental Science, 2(1): 12025 |
Ikehata K, Notsu K and Hirata T. 2008. In situ determination of Cu isotope ratios in copper-rich materials by NIR femtosecond LA-MC-ICP-MS. Journal of Analytical Atomic Spectrometry, 23(7): 1003-1008 DOI:10.1039/b801044g |
Ikehata K, Notsu K and Hirata T. 2011. Copper isotope characteristics of copper-rich minerals from Besshi-type volcanogenic massive sulfide deposits, Japan, determined using a femtosecond LA-MC-ICP-MS. Economic Geology, 106(2): 307-316 DOI:10.2113/econgeo.106.2.307 |
Ikehata K and Hirata T. 2012. Copper isotope characteristics of copper-rich minerals from the Horoman peridotite complex, Hokkaido, northern Japan. Economic Geology, 107(7): 1489-1497 DOI:10.2113/econgeo.107.7.1489 |
Ikehata K and Hirata T. 2013. Evaluation of UV-fs-LA-MC-ICP-MS for precise in situ copper isotopic microanalysis of cubanite. Analytical Sciences, 29(12): 1213-1217 DOI:10.2116/analsci.29.1213 |
Jackson SE and Günther D. 2003. The nature and sources of laser induced isotopic fractionation in laser ablation-multicollector-inductively coupled plasma-mass spectrometry. Journal of Analytical Atomic Spectrometry, 18(3): 205-212 DOI:10.1039/b209620j |
John SG, Rouxel OJ, Craddock PR, Engwall AM and Boyle EA. 2008. Zinc stable isotopes in seafloor hydrothermal vent fluids and chimneys. Earth and Planetary Science Letters, 269(1-2): 17-28 DOI:10.1016/j.epsl.2007.12.011 |
John SG and Conway TM. 2014. A role for scavenging in the marine biogeochemical cycling of zinc and zinc isotopes. Earth and Planetary Science Letters, 394: 159-167 DOI:10.1016/j.epsl.2014.02.053 |
Johnson CM, Beard BL, Beukes NJ, Klein C and O'Leary JM. 2003. Ancient geochemical cycling in the Earth as inferred from Fe isotope studies of banded iron formations from the Transvaal Craton. Contributions to Mineralogy and Petrology, 144(5): 523-547 DOI:10.1007/s00410-002-0418-x |
Johnson CM, Bear BL, Roden EE, Newman DK and Nealson KH. 2004. Isotopic constraints on biogeochemical cycling of Fe. Reviews in Mineralogy and Geochemistry, 55(1): 359-408 DOI:10.2138/gsrmg.55.1.359 |
Johnson CM and Beard BL. 2006. Fe isotopes: An emerging technique for understanding modern and ancient biogeochemical cycles. GSA Today, 16(11): 4-10 DOI:10.1130/GSAT01611A.1 |
Johnson CM, Beard BL, Klein C, Beukes NJ and Roden EE. 2008. Iron isotopes constrain biologic and abiologic processes in banded iron formation genesis. Geochimica et Cosmochimica Acta, 72(1): 151-169 DOI:10.1016/j.gca.2007.10.013 |
Kelley KD, Wilkinson JJ, Chapman JB, Crowther H and Weiss DJ. 2009. Zinc isotopes in sphalerite from base metal deposits in the Red Dog District, northern Alaska. Economic Geology, 104(6): 767-773 DOI:10.2113/gsecongeo.104.6.767 |
Kimball BE, Mathur R, Dohnalkova AC, Wall AJ, Runkel RL and Brantley SL. 2009. Copper isotope fractionation in acid mine drainage. Geochimica et Cosmochimica Acta, 73(5): 1247-1263 DOI:10.1016/j.gca.2008.11.035 |
Kuhn HR, Pearson NJ and Jackson SE. 2007. The influence of the laser ablation process on isotopic fractionation of copper in LA-MC-ICP-MS. Journal of Analytical Atomic Spectrometry, 22(5): 547-552 DOI:10.1039/b616232k |
Larson PB, Maher K, Ramos FC, Chang ZS, Gaspar M and Meinert LD. 2003. Copper isotope ratios in magmatic and hydrothermal ore-forming environments. Chemical Geology, 201(3-4): 337-350 DOI:10.1016/j.chemgeo.2003.08.006 |
Leach DL, Marsh E, Emsbo P, Rombach CS, Kelley KD and Anthony M. 2004. Nature of hydrothermal fluids at the shale-hosted red dog Zn-Pb-Ag deposits, Brooks Range, Alaska. Economic Geology, 99(7): 1449-1480 DOI:10.2113/gsecongeo.99.7.1449 |
Li ML, Liu SA, Xue CJ and Li DD. 2019. Zinc, cadmium and sulfur isotope fractionation in a supergiant MVT deposit with bacteria. Geochimica et Cosmochimica Acta, 265: 1-18 DOI:10.1016/j.gca.2019.08.018 |
Li WQ, Jackson SE, Pearson NJ, Alard O and Chappell BW. 2009. The Cu isotopic signature of granites from the Lachlan Fold Belt, SE Australia. Chemical Geology, 258(1-2): 38-49 DOI:10.1016/j.chemgeo.2008.06.047 |
Li WQ, Jackson SE, Pearson NJ and Graham S. 2010. Copper isotopic zonation in the Northparkes porphyry Cu-Au deposit, SE Australia. Geochimica et Cosmochimica Acta, 74(14): 4078-4096 DOI:10.1016/j.gca.2010.04.003 |
Li ZH, Zhu XK and Tang SH. 2008a. Characters of Fe isotopes and rare earth elements of banded iron formations from Anshan-Benxi area: Implications for Fe source. Acta Petrologica et Mineralogica, 27(4): 285-290 (in Chinese with English abstract) |
Li ZH, Zhu XK, Tang SH and Li YH. 2008b. Fe isotope fractionation between magnetite and pyrite during green schist-lower amphibolite facies metamorphism. Acta Petrologica et Mineralogica, 27(4): 291-297 (in Chinese with English abstract) |
Li ZH and Zhu XK. 2012. Geochemical features of Xuanlong type iron ore deposit in Hebei Province and their geological significances. Acta Petrologica Sinica, 28(9): 2903-2911 (in Chinese with English abstract) |
Little SH, Vance D, Walker-Brown C and Landing WM. 2014. The oceanic mass balance of copper and zinc isotopes, investigated by analysis of their inputs, and outputs to ferromanganese oxide sediments. Geochimica et Cosmochimica Acta, 125: 673-693 DOI:10.1016/j.gca.2013.07.046 |
Liu SA, Li DD, Li SG, Teng FZ, Ke S, He YS and Lu YH. 2014. High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. Journal of Analytical Atomic Spectrometry, 29(1): 122-133 DOI:10.1039/C3JA50232E |
Liu SA, Huang J, Liu JA, Wörner G, Yang W, Tang YJ, Chen Y, Tang LM, Zheng JP and Li SG. 2015. Copper isotopic composition of the silicate Earth. Earth and Planetary Science Letters, 427: 95-103 DOI:10.1016/j.epsl.2015.06.061 |
Maher KC and Larson PB. 2007. Variation in copper isotope ratios and controls on fractionation in hypogene skarn mineralization at Coroccohuayco and Tintaya, Peru′. Economic Geology, 102(2): 225-237 DOI:10.2113/gsecongeo.102.2.225 |
Maher KC, Jackson S and Mountain B. 2011. Experimental evaluation of the fluid-mineral fractionation of Cu isotopes at 250℃ and 300℃. Chemical Geology, 286(3-4): 229-239 |
Malitch KN, Latypov RM, Badanina IY and Sluzhenikin SF. 2014. Insights into ore genesis of Ni-Cu-PGE sulfide deposits of the Noril'sk Province (Russia): Evidence from copper and sulfur isotopes. Lithos, 204: 172-187 DOI:10.1016/j.lithos.2014.05.014 |
Maréchal CN, Télouk P and Albarède F. 1999. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chemical Geology, 156(1-4): 251-273 DOI:10.1016/S0009-2541(98)00191-0 |
Maréchal CN, Nicolas E, Douchet C and Albarède F. 2000. Abundance of zinc isotopes as a marine biogeochemical tracer. Geochemistry, Geophysics, Geosystems, 1(5): 1015 |
Maréchal CN and Albarède F. 2002. Ion-exchange fractionation of copper and zinc isotopes. Geochimica et Cosmochimica Acta, 66(9): 1499-1509 DOI:10.1016/S0016-7037(01)00815-8 |
Maréchal CN and Sheppard SMF. 2002. Isotopic fractionation of Cu and Zn between chloride and nitrate solutions and malachite or smithsonite at 30℃ and 50℃. Geochimica et Cosmochimica Acta, 66(15A): A484 |
Markl G, Lahaye Y and Schwinn G. 2006a. Copper isotopes as monitors of redox processes in hydrothermal mineralization. Geochimica et Cosmochimica Acta, 70(16): 4215-4228 DOI:10.1016/j.gca.2006.06.1369 |
Markl G, Von Blanckenburg F and Wagner T. 2006b. Iron isotope fractionation during hydrothermal ore deposition and alteration. Geochimica et Cosmochimica Acta, 70(12): 3011-3030 DOI:10.1016/j.gca.2006.02.028 |
Mason TFD, Weiss DJ, Horstwood M, Parrish RR, Russell SS, Mullane E and Coles BJ. 2004. High-precision Cu and Zn isotope analysis by plasma source mass spectrometry Part 1. Spectral interferences and their correction. Journal of Analytical Atomic Spectrometry, 19(2): 209-217 DOI:10.1039/b306958c |
Mason TFD, Weiss DJ, Chapman JB, Wilkinson JJ, Tessalina SG, Spiro B, Horstwood MSA, Spratt J and Coles BJ. 2005. Zn and Cu isotopic variability in the Alexandrinka volcanic-hosted massive sulphide (VHMS) ore deposit, Urals, Russia. Chemical Geology, 221(3-4): 170-187 DOI:10.1016/j.chemgeo.2005.04.011 |
Mathur R, Ruiz J, Titley S, Liermann L, Buss H and Brantley S. 2005. Cu isotopic fractionation in the supergene environment with and without bacteria. Geochimica et Cosmochimica Acta, 69(22): 5233-5246 DOI:10.1016/j.gca.2005.06.022 |
Mathur R, Titley S, Barra F, Brantley S, Wilson M, Phillips A, Munizaga F, Maksaev V, Vervoort J and Hart G. 2009. Exploration potential of Cu isotope fractionation in porphyry copper deposits. Journal of Geochemical Exploration, 102(1): 1-6 DOI:10.1016/j.gexplo.2008.09.004 |
Mathur R and Schlitt WJ. 2010. Identification of the dominant Cu ore minerals providing soluble copper at Cañariaco, Peru through Cu isotope analyses of batch leach experiments. Hydrometallurgy, 101(1-2): 15-19 DOI:10.1016/j.hydromet.2009.11.005 |
Mathur R, Dendas M, Titley S and Phillips A. 2010. Patterns in the copper isotope composition of minerals in porphyry copper deposits in Southwestern United States. Economic Geology, 105(8): 1457-1467 DOI:10.2113/econgeo.105.8.1457 |
Mathur R, Jin L, Prush V, Paul J, Ebersole C, Fornadel A, Williams JZ and Brantley S. 2012. Cu isotopes and concentrations during weathering of black shale of the Marcellus Formation, Huntingdon County, Pennsylvania (USA). Chemical Geology, 304-305: 175-184 DOI:10.1016/j.chemgeo.2012.02.015 |
Mathur R, Munk L, Nguyen M, Gregory M, Annell H and Lang J. 2013. Modern and paleofluid pathways revealed by Cu isotope compositions in surface waters and ores of the Pebble porphyry Cu-Au-Mo deposit, Alaska. Economic Geology, 108(3): 529-541 DOI:10.2113/econgeo.108.3.529 |
Mathur R, Munk LA, Townley B, Gou KY, Miguélez NG, Titley S, Chen GG, Song S, Reich M, Tornos F and Ruiz J. 2014. Tracing low-temperature aqueous metal migration in mineralized watersheds with Cu isotope fractionation. Applied Geochemistry, 51: 109-115 DOI:10.1016/j.apgeochem.2014.09.019 |
Mathur R and Fantle MS. 2015. Copper isotopic perspectives on supergene processes: Implications for the global cu cycle. Elements, 11(5): 323-329 DOI:10.2113/gselements.11.5.323 |
Meng XJ, Yang ZS, Qi XX, Hou ZQ and Li ZQ. 2008. Silicon-oxygen-hydrogen isotopic compositions of Zhaxikang antimony polymetallic deposit in Southern Tibet and its responses to the ore-controlling structure. Acta Petrologica Sinica, 24(7): 1649-1655 (in Chinese with English abstract) |
Metz S and Trefry JH. 2000. Chemical and mineralogical influences on concentrations of trace metals in hydrothermal fluids. Geochimica et Cosmochimica Acta, 64(13): 2267-2279 DOI:10.1016/S0016-7037(00)00354-9 |
Mirnejad H, Mathur R, Einali M, Dendas M and Alirezaei S. 2010. A comparative copper isotope study of porphyry copper deposits in Iran. Geochemistry: Exploration, Environment, Analysis, 10(4): 413-418 DOI:10.1144/1467-7873/09-229 |
Moynier F, Blichert-Toft J, Telouk P, Luck JM and Albarède F. 2007. Comparative stable isotope geochemistry of Ni, Cu, Zn, and Fe in chondrites and iron meteorites. Geochimica et Cosmochimica Acta, 71(17): 4365-4379 DOI:10.1016/j.gca.2007.06.049 |
Navarrete JU, Borrok DM, Viveros M and Ellzey JT. 2011a. Copper isotope fractionation during surface adsorption and intracellular incorporation by bacteria. Geochimica et Cosmochimica Acta, 75(3): 784-799 DOI:10.1016/j.gca.2010.11.011 |
Navarrete JU, Viveros M, Ellzey JT and Borrok DM. 2011b. Copper isotope fractionation by desert shrubs. Applied Geochemistry, 26(Suppl.): S319-S321 DOI:10.1016/j.apgeochem.2011.04.002 |
Palacios C, Rouxel O, Reich M, Cameron EM and Leybourne MI. 2011. Pleistocene recycling of copper at a porphyry system, Atacama Desert, Chile: Cu isotope evidence. Mineralium Deposita, 46(1): 1-7 DOI:10.1007/s00126-010-0315-6 |
Peel K, Weiss D, Chapman J, Arnold T and Coles B. 2008. A simple combined sample-standard bracketing and inter-element correction procedure for accurate mass bias correction and precise Zn and Cu isotope ratio measurements. Journal of Analytical Atomic Spectrometry, 23(1): 103-110 DOI:10.1039/B710977F |
Petit JC, De Jong J, Chou L and Mattielli N. 2008. Development of Cu and Zn Isotope MC-ICP-MS measurements: Application to suspended particulate matter and sediments from the scheldt estuary. Geostandards and Geoanalytical Research, 32(2): 149-166 DOI:10.1111/j.1751-908X.2008.00867.x |
Pichat S, Douchet C and Albarède F. 2003. Zinc isotope variations in deep-sea carbonates from the eastern equatorial Pacific over the last 175ka. Earth and Planetary Science Letters, 210(1-2): 167-178 DOI:10.1016/S0012-821X(03)00106-7 |
Planavsky N, Rouxel O, Bekker A, Shapiro R, Fralick P and Knudsen A. 2009. Iron-oxidizing microbial ecosystems thrived in late Paleoproterozoic redox-stratified oceans. Earth and Planetary Science Letters, 286(1-2): 230-242 DOI:10.1016/j.epsl.2009.06.033 |
Planavsky N, Rouxel OJ, Bekker A, Hofmann A, Little CTS and Lyons TW. 2012. Iron isotope composition of some Archean and Proterozoic iron formations. Geochimica et Cosmochimica Acta, 80: 158-169 DOI:10.1016/j.gca.2011.12.001 |
Poitrasson F, Halliday AN, Lee DC, Levasseur S and Teutsch N. 2004. Iron isotope differences between Earth, Moon, Mars and Vesta as possible records of contrasted accretion mechanisms. Earth and Planetary Science Letters, 223(3-4): 253-266 DOI:10.1016/j.epsl.2004.04.032 |
Poitrasson F and Freydier R. 2005. Heavy iron isotope composition of granites determined by high resolution MC-ICP-MS. Chemical Geology, 222(1-2): 132-147 DOI:10.1016/j.chemgeo.2005.07.005 |
Pokrovsky OS, Viers J, Emnova EE, Kompantseva EI and Freydier R. 2008. Copper isotope fractionation during its interaction with soil and aquatic microorganisms and metal oxy(hydr)oxides: Possible structural control. Geochimica et Cosmochimica Acta, 72(7): 1742-1757 DOI:10.1016/j.gca.2008.01.018 |
Pribil MJ, Wanty RB, Ridley WI and Borrok DM. 2009. Influence of sulfur-bearing polyatomic species on high precision measurements of Cu isotopic composition. Chemical Geology, 272(1-4): 49-54 |
Ripley EM, Dong SF, Li CS and Wasylenki LE. 2015. Cu isotope variations between conduit and sheet-style Ni-Cu-PGE sulfide mineralization in the Midcontinent Rift System, North America. Chemical Geology, 414: 59-68 DOI:10.1016/j.chemgeo.2015.09.007 |
Rouxel O, Fouquet Y and Ludden JN. 2004. Copper isotope systematics of the lucky strike, rainbow, and logatchev sea-floor hydrothermal fields on the Mid-Atlantic Ridge. Economic Geology, 99(3): 585-600 DOI:10.2113/gsecongeo.99.3.585 |
Rouxel O, Shanks Ⅲ WC, Bach W and Edwards KJ. 2008. Integrated Fe- and S-isotope study of seafloor hydrothermal vents at East Pacific Rise 9~10°N. Chemical Geology, 252(3-4): 214-227 DOI:10.1016/j.chemgeo.2008.03.009 |
Rouxel OJ, Bekker A and Edwards KJ. 2005. Iron isotope constraints on the Archean and Paleoproterozoic ocean redox state. Science, 307(5712): 1088-1091 DOI:10.1126/science.1105692 |
Saunders JA, Mathur R, Kamenov GD, Shimizu T and Brueseke ME. 2016. New isotopic evidence bearing on bonanza (Au-Ag) epithermal ore-forming processes. Mineralium Deposita, 51(1): 1-11 DOI:10.1007/s00126-015-0623-y |
Schuessler JA, Schoenberg R and Sigmarsson O. 2009. Iron and lithium isotope systematics of the Hekla volcano, Iceland: Evidence for Fe isotope fractionation during magma differentiation. Chemical Geology, 258(1-2): 78-91 DOI:10.1016/j.chemgeo.2008.06.021 |
Seewald JS and Seyfried Jr WE. 1990. The effect of temperature on metal mobility in subseafloor hydrothermal systems: Constraints from basalt alteration experiments. Earth and Planetary Science Letters, 101(2-4): 388-403 DOI:10.1016/0012-821X(90)90168-W |
Severmann S, Johnson CM, Beard BL, German CR, Edmonds HN, Chiba H and Green DRH. 2004. The effect of plume processes on the Fe isotope composition of hydrothermally derived Fe in the deep ocean as inferred from the Rainbow vent site, Mid-Atlantic ridge, 36°14′N. Earth and Planetary Science Letters, 225(1-2): 63-76 DOI:10.1016/j.epsl.2004.06.001 |
Severmann S, Johnson CM, Beard BL and McManus J. 2006. The effect of early diagenesis on the Fe isotope compositions of porewaters and authigenic minerals in continental margin sediments. Geochimica et Cosmochimica Acta, 70(8): 2006-2022 DOI:10.1016/j.gca.2006.01.007 |
Shafiei B, Shamanian G, Mathur R and Mirnejad H. 2015. Mo isotope fractionation during hydrothermal evolution of porphyry Cu systems. Mineralium Deposita, 50(3): 281-291 DOI:10.1007/s00126-014-0537-0 |
Sharma M, Polizzotto M and Anbar AD. 2001. Iron isotopes in hot springs along the Juan de Fuca Ridge. Earth and Planetary Science Letters, 194(1-2): 39-51 DOI:10.1016/S0012-821X(01)00538-6 |
Song SM, Mathur R, Ruiz J, Chen Dd, Allin N, Guo KY and Kang WK. 2016. Fingerprinting two metal contaminants in streams with Cu isotopes near the Dexing Mine, China. Science of the Total Environment, 544: 677-685 DOI:10.1016/j.scitotenv.2015.11.101 |
Sossi PA, Halverson GP, Nebel O and Eggins SM. 2015. Combined separation of Cu, Fe and Zn from rock matrices and improved analytical protocols for stable isotope determination. Geostandards and Geoanalytical Research, 39(2): 129-149 DOI:10.1111/j.1751-908X.2014.00298.x |
Steinhoefel G, Horn I and von Blanckenburg F. 2009. Micro-scale tracing of Fe and Si isotope signatures in banded iron formation using femtosecond laser ablation. Geochimica et Cosmochimica Acta, 73(18): 5343-5360 DOI:10.1016/j.gca.2009.05.037 |
Steinhoefel G, von Blanckenburg F, Horn I, Konhauser KO, Beukes NJ and Gutzmer J. 2010. Deciphering formation processes of banded iron formations from the Transvaal and the Hamersley successions by combined Si and Fe isotope analysis using UV femtosecond laser ablation. Geochimica et Cosmochimica Acta, 74(9): 2677-2696 DOI:10.1016/j.gca.2010.01.028 |
Stock E. 2007. Footwall veins in the Red Dog district, Brooks Range, Alaska: Fluid conduits for giant SEDEX ore deposits. Master Degree Thesis. Vancouver: British Columbia University, 1-81
|
Sun J, Zhu XK, Chen YL and Fang N. 2013. Iron isotopic constraints on the genesis of Bayan Obo ore deposit, Inner Mongolia, China. Precambrian Research, 235: 88-106 DOI:10.1016/j.precamres.2013.06.004 |
Taylor PDP, Maeck R and De Bièvre P. 1992. Determination of the absolute isotopic composition and atomic weight of a reference sample of natural iron. International Journal of Mass Spectrometry and Ion Processes, 121(1-2): 111-125 DOI:10.1016/0168-1176(92)80075-C |
Telus M, Dauphas N, Moynier F, Tissot FLH, Teng FZ, Nabelek PI, Craddock PR and Groat LA. 2012. Iron, zinc, magnesium and uranium isotopic fractionation during continental crust differentiation: The tale from migmatites, granitoids, and pegmatites. Geochimica et Cosmochimica Acta, 97: 247-265 DOI:10.1016/j.gca.2012.08.024 |
Toutain JP, Sonke J, Munoz M, Nonell A, Polvé M, Viers J, Freydier R, Sortino F, Joron JL and Sumarti S. 2008. Evidence for Zn isotopic fractionation at Merapi volcano. Chemical Geology, 253(1-2): 74-82 DOI:10.1016/j.chemgeo.2008.04.007 |
Tsikos H, Matthews A, Erel Y and Moore JM. 2010. Iron isotopes constrain biogeochemical redox cycling of iron and manganese in a Palaeoproterozoic stratified basin. Earth and Planetary Science Letters, 298(1-2): 125-134 DOI:10.1016/j.epsl.2010.07.032 |
Vance D, Archer C, Bermin J, Perkins J, Statham PJ, Lohan MC, Ellwood MJ and Mills RA. 2008. The copper isotope geochemistry of rivers and the oceans. Earth and Planetary Science Letters, 274(1-2): 204-213 DOI:10.1016/j.epsl.2008.07.026 |
Viers J, Oliva P, Nonell A, Gélabert A, Sonke JE, Freydier R, Gainville R and Dupré B. 2007. Evidence of Zn isotopic fractionation in a soil-plant system of a pristine tropical watershed (Nsimi, Cameroon). Chemical Geology, 239(1-2): 124-137 DOI:10.1016/j.chemgeo.2007.01.005 |
Von Blanckenburg F, Mamberti M, Schoenberg R, Kamber BS and Webb GE. 2008. The iron isotope composition of microbial carbonate. Chemical Geology, 249(1-2): 113-128 DOI:10.1016/j.chemgeo.2007.12.001 |
Wall AJ, Heaney PJ, Mathur R, Post JE, Hanson JC and Eng PJ. 2011a. A flow-through reaction cell that couples time-resolved X-ray diffraction with stable isotope analysis. Journal of Applied Crystallography, 44(2): 429-432 DOI:10.1107/S0021889811000525 |
Wall AJ, Mathur R, Post JE and Heaney PJ. 2011b. Cu isotope fractionation during bornite dissolution: An in situ X-ray diffraction analysis. Ore Geology Reviews, 42(1): 62-70 DOI:10.1016/j.oregeorev.2011.01.001 |
Wang D, Sun X, Zheng YY, Wu S, Xia SL, Chang HF and Yu M. 2017b. Two pulses of mineralization and genesis of the Zhaxikang Sb-Pb-Zn-Ag deposit in southern Tibet: Constraints from Fe-Zn isotopes. Ore Geology Reviews, 84: 347-363 DOI:10.1016/j.oregeorev.2016.12.030 |
Wang D, Zheng YY, Mathur R and Wu S. 2018. The Fe-Zn isotopic characteristics and fractionation models: Implications for the genesis of the Zhaxikang Sb-Pb-Zn-Ag deposit in Southern Tibet. Geofluids: 2197891 DOI:10.1155/2018/2197891 |
Wang P, Dong GC, Santosh M, Liu KR and Li XF. 2017a. Copper isotopes trace the evolution of skarn ores: A case study from the Hongshan-Hongniu Cu deposit, southwest China. Ore Geology Reviews, 88: 822-831 DOI:10.1016/j.oregeorev.2016.11.023 |
Wang Y and Zhu XK. 2010a. Application of Zn isotopes to study of mineral deposits: A review. Mineral Deposits, 29(5): 843-852 (in Chinese with English abstract) |
Wang Y and Zhu XK. 2010b. Applications of Cu isotopes on studies of mineral deposits: A status report. Journal of Jilin University (Earth Science Edition), 40(4): 739-751 (in Chinese with English abstract) |
Wang Y, Zhu XK, Mao JW, Li ZH and Cheng YB. 2011. Iron isotope fractionation during skarn-type metallogeny: A case study of Xinqiao Cu-S-Fe-Au deposit in the Middle-Lower Yangtze valley. Ore Geology Review, 43(1): 194-202 DOI:10.1016/j.oregeorev.2010.12.004 |
Wang Y and Zhu XK. 2012. Fe isotope systematics and its implications in ore deposit geology. Acta Petrologica Sinica, 28(11): 3638-3654 (in Chinese with English abstract) |
Wang Y, Zhu XK and Cheng YB. 2015. Fe isotope behaviours during sulfide-dominated skarn-type mineralisation. Journal of Asian Earth Sciences, 103: 374-392 DOI:10.1016/j.jseaes.2014.11.005 |
Wawryk CM and Foden JD. 2015. Fe-isotope fractionation in magmatic-hydrothermal mineral deposits: A case study from the Renison Sn-W deposit, Tasmania. Geochimica et Cosmochimica Acta, 150: 285-298 DOI:10.1016/j.gca.2014.09.044 |
Weiss DJ, Rausch N, Mason TFD, Coles BJ, Wilkinson JJ, Ukonmaanaho L, Arnold T and Nieminen TM. 2007. Atmospheric deposition and isotope biogeochemistry of zinc in ombrotrophic peat. Geochimica et Cosmochimica Acta, 71(14): 3498-3517 DOI:10.1016/j.gca.2007.04.026 |
Whitehouse MJ and Fedo CM. 2007. Microscale heterogeneity of Fe isotopes in >3. 71Ga banded iron formation from the Isua Greenstone Belt, southwest Greenland. Geology, 35(8): 719-722 |
Wilkinson JJ, Weiss DJ, Mason TFD and Coles BJ. 2005. Zinc isotope variation in hydrothermal systems: Preliminary evidence from the Irish Midlands ore field. Economic Geology, 100(3): 583-590 DOI:10.2113/gsecongeo.100.3.583 |
Wilson M, Wilson DL and Mathur R. 2016. Tracing the source of native copper mineral specimens with copper isotope values. Rocks & Minerals, 91(4): 352-356 DOI:10.1080/00357529.2016.1172176 |
Wu LY, Hu RZ, Li XF, Liu SA, Tang YW and Tang YY. 2017b. Copper isotopic compositions of the Zijinshan high-sulfidation epithermal Cu-Au deposit, South China: Implications for deposit origin. Ore Geology Reviews, 83: 191-199 DOI:10.1016/j.oregeorev.2016.12.013 |
Wu S, Zheng YY, Wang D, Chang HF and Tan M. 2017a. Variation of copper isotopes in chalcopyrite from Dabu porphyry Cu-Mo deposit in Tibet and implications for mineral exploration. Ore Geology Reviews, 90: 14-24 DOI:10.1016/j.oregeorev.2017.10.001 |
Xie YL, Li LM, Wang BG, Li GM, Liu HF, Li YX, Dong SL and Zhou JJ. 2017. Genesis of the Zhaxikang epithermal Pb-Zn-Sb deposit in southern Tibet, China: Evidence for a magmatic link. Ore Geology Reviews, 80: 891-909 DOI:10.1016/j.oregeorev.2016.08.007 |
Yamaguchi KE, Johnson CM, Beard BL and Ohmoto H. 2005. Biogeochemical cycling of iron in the Archean-Paleoproterozoic Earth: Constraints from iron isotope variations in sedimentary rocks from the Kaapvaal and Pilbara Cratons. Chemical Geology, 218(1-2): 135-169 DOI:10.1016/j.chemgeo.2005.01.020 |
Yan B, Zhu XK, Tang SH and Zhu MY. 2010. Fe isotopic characteristics of the Neoproterozoic BIF in Guangxi Province and its implications. Acta Geologica Sinica, 84(7): 1080-1086 (in Chinese with English abstract) |
Yao JM, Mathur R, Sun WD, Song WL, Chen HY, Mutti L, Xiang XK and Luo XH. 2016. Fractionation of Cu and Mo isotopes caused by vapor-liquid partitioning, evidence from the Dahutang W-Cu-Mo ore field. Geochemistry, Geophysics, Geosystems, 17(5): 1725-1739 DOI:10.1002/2016GC006328 |
Zhang JF, Zheng YY, Zhang GY, Gao SB, Ye XR, Zhang Z, Liu MY and Li JQ. 2010. Genesis of Zhaxikang Pb-Zn-Sb-Ag deposit in northern Himalaya: constraints from multi-isotope geochemistry. Earth Science (Journal of China University of Geosciences), 35(6): 1000-1010 (in Chinese with English abstract) DOI:10.3799/dqkx.2010.113 |
Zhao Y, Vance D, Abouchami W and De Baar HJW. 2014. Biogeochemical cycling of zinc and its isotopes in the Southern Ocean. Geochimica et Cosmochimica Acta, 125: 653-672 DOI:10.1016/j.gca.2013.07.045 |
Zhao Y, Xue CJ, Liu SA, Symons DTA, Zhao XB, Yang YQ and Ke JJ. 2017. Copper isotope fractionation during sulfide-magma differentiation in the Tulaergen magmatic Ni-Cu deposit, NW China. Lithos, 286-287: 206-215 DOI:10.1016/j.lithos.2017.06.007 |
Zheng YY, Liu MY, Sun X, Yuan EH, Tian LM, Zheng HT, Zhang GY and Zhang LH. 2012. Type, discovery process and significance of Zhaxikang antimony polymetallic ore deposit, Tibet. Earth Science (Journal of China University of Geosciences), 37(5): 1003-1014 (in Chinese with English abstract) |
Zhou JX, Huang ZL, Zhou MF, Zhu XK and Muchez P. 2014. Zinc, sulfur and lead isotopic variations in carbonate-hosted Pb-Zn sulfide deposits, Southwest China. Ore Geology Review, 58: 41-54 DOI:10.1016/j.oregeorev.2013.10.009 |
Zhou Q, Li WC, Qing CS, Lai Y, Li YX, Liao ZW, Wu JY, Wang SW, Dong L and Tian EY. 2018. Origin and tectonic implications of the Zhaxikang Pb-Zn-Sb-Ag deposit in northern Himalaya: Evidence from structures, Re-Os-Pb-S isotopes, and fluid inclusions. Mineralium Deposita, 53(4): 585-600 DOI:10.1007/s00126-017-0760-6 |
Zhu B, Zhang HF, Zhao XM and He YS. 2016. Iron isotope fractionation during skarn-type alteration: Implications for metal source in the Han-Xing iron skarn deposit. Ore Geology Review, 74: 139-150 DOI:10.1016/j.oregeorev.2015.11.001 |
Zhu XK, O'Nions RK, Guo Y, Belshaw NS and Rickard D. 2000. Determination of natural Cu-isotope variation by plasma-source mass spectrometry: Implications for use as geochemical tracers. Chemical Geology, 163(1-4): 139-149 DOI:10.1016/S0009-2541(99)00076-5 |
Zhu XK, Guo Y, Williams RJP, O'Nions RK, Matthews A, Belshaw NS, Canters GW, de Waal EC, Weser U, Burgess BK and Salvato B. 2002. Mass fractionation processes of transition metal isotopes. Earth and Planetary Science Letters, 200(1-2): 47-62 DOI:10.1016/S0012-821X(02)00615-5 |
Zhu XK, Li ZH, Tang SH and Li YH. 2008. Fe isotope characteristics of Early Precambrian pyrite deposits and their geological significance: Examples from Shandong and Hebei provinces. Acta Petrologica et Mineralogica, 27(5): 429-434 (in Chinese with English abstract) |
李志红, 朱祥坤, 唐索寒. 2008a. 鞍山-本溪地区条带状铁建造的铁同位素与稀土元素特征及其对成矿物质来源的指示. 岩石矿物学杂志, 27(4): 285-290. |
李志红, 朱祥坤, 唐索寒, 李延河. 2008b. 绿片岩-低角闪岩相变质条件下磁铁铁矿与黄铁矿间的Fe同位素分馏. 岩石矿物学杂志, 27(4): 291-297. |
李志红, 朱祥坤. 2012. 河北省宣龙式铁矿的地球化学特征及其地质意义. 岩石学报, 28(9): 2903-2911. |
孟祥金, 杨竹森, 戚学祥, 侯增谦, 李振清. 2008. 藏南扎西康锑多金属矿硅-氧-氢同位素组成及其对成矿构造控制的响应. 岩石学报, 24(7): 1649-1655. |
王跃, 朱祥坤. 2010a. 锌同位素在矿床学中的应用:认识与进展. 矿床地质, 29(5): 843-852. |
王跃, 朱祥坤. 2010b. 铜同位素在矿床学中的应用:认识与进展. 吉林大学学报(地球科学版), 40(4): 739-751. |
王跃, 朱祥坤. 2012. 铁同位素体系及其在矿床学中的应用. 岩石学报, 28(11): 3638-3654. |
闫斌, 朱祥坤, 唐索寒, 朱茂炎. 2010. 广西新元古代BIF的铁同位素特征及其地质意义. 地质学报, 84(7): 1080-1086. |
张建芳, 郑有业, 张刚阳, 高顺宝, 叶先仁, 张众, 刘敏院, 李及秋. 2010. 北喜马拉雅扎西康铅锌锑银矿床成因的多元同位素制约. 地球科学(中国地质大学学报), 35(6): 1000-1010. |
郑有业, 刘敏院, 孙祥, 原恩会, 田立明, 郑海涛, 张刚阳, 张立华. 2012. 西藏扎西康锑多金属矿床类型、发现过程及意义. 地球科学(中国地质大学学报), 37(5): 1003-1014. |
朱祥坤, 李志红, 唐索寒, 李延河. 2008. 早前寒武纪硫铁矿矿床Fe同位素特征及其地质意义——以山东石河庄和河北大川为例. 岩石矿物学杂志, 27(5): 429-434. |
 2020, Vol. 36
2020, Vol. 36