2. Department of Thoracic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China;
3. Department of International Education, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
Song Tong, M.D. (Department of Neurosurgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 2014) and PhD (Department of Thoracic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 2017). He has published four SCI papers.
Ahmed Abdelmaksoud, MSc. (Benha University, School of Medicine); Egyptian Doctorate student in Neurosurgery (Wuhan Union Hospital, Tongji, Medical College, Huazhong University of Science and Technology, China).
Fu Peng, Attending Doctor (Department of Neurosurgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan); MSc. in Neurosurgery (Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; 2006–2009); PhD in Neurosurgery (Klinikum Großhadern, Ludwig Maximillian University, Munich, Germany; 2009–2012). Scientific and Clinical Specialty: experimental and clinical neuro-oncology, functional neurosurgery (both surgery and radiosurgery).
Huang Tao, PhD candidate of Neurosurgery since 2016 (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China); Master degree of Neurosurgery (2013; Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China); Bachelor degree of Clinical Medicine (2010; Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China); Neurosurgery resident (2013–2016; Department of Neurosurgery, Puai Hospital, Wuhan, China). Current research interests: regenerative medicine, spinal cord injury.
Yizhi Huang, Master degree candidate in Neurosurgery since 2014 (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China); Bachelor degree (2014; Yangtze University). Current research interests: axon regeneration.
Weichao Liu, Master degree candidate in Neurosurgery (Department of Neurosurgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China); Bachelor of Medicine (2016; Zhengzhou University).
Yuan Ye, Master degree candidate (Department of Neurosurgery, Union Hospital, Tongji Medical College Huazhong University of Science and Technology, Wuhan, China); Bachelor of Medicine (2016; Henan University of Science and Technology). Publications: Yuan et al. (2015) An UPLC-MS/MS method for the analysis of glimepiride and fluoxetine in human plasma. Journal of Chromatography B, 980: 16-19.
Raya Ahmad Almaraihah, Undergraduate MBBS student at the Union hospital. She has volunteered at the United Nations, United States Agency for International Development, and the Mercy Corps.
Nanxiang Xiong, Professor, PhD supervisor, and Vice president at the Department of Neurosurgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology; PhD (2005; Department of Neurosurgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology). He is currently conducting two projects that are funded by the national natural science and technology. He has published more than 10 SCI papers.
Chronic subdural hematomas (CSDH) are the most benign of all the intracranial hematomas with a mortality rate of 0.5%–4.0%[1, 2]. Burr-hole craniotomy appears to be the most common surgical treatment for evacuation of CSDH. The main surgical complications of this procedure include hematoma recurrence, tension pneumocephalus, supratentorial hemorrhage, and intracranial hypotension[3]. Here, we reported the first case in the literature of CSDH with one side undeveloped sigmoid sinus and internal jugular vein, complicated by severe bilateral symmetric remote cerebellar hemorrhage (RCH; diameter >4 cm) and right side frontal lobe hemorrhage after burr-hole evacuation. Further, we discussed possible etiologic mechanisms.
2 Case reportA 64-year-old man presented with a 2-month history of increasing headache, confusion, and progressive weakness on the left side limbs (grade 4/5). He had a past medical history of stent implantation owing to coronary heart disease and used aspirin as anti-platelet therapy. He had stopped the anti-platelet therapy one month prior owing to hematuria, which disappeared after cessation of aspirin. Brain computed tomography (CT) was performed after admission to our hospital, which revealed a hematoma with slight hyperdensity leading to a midline shift with compression of the right ventricle. The volume of the subdural hematoma was approximately 100 mL (Figure 1).
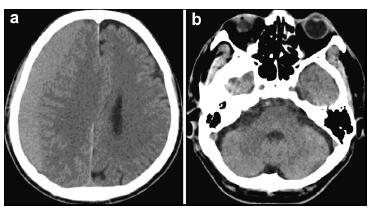
|
| Figure 1 Preoperative computed tomography images of the brain show a right side subdural hematoma without abnormal findings in the posterior fossa and right side frontal lobe. |
Anti-platelet therapy was suspected as a possible cause of the spontaneous occurrence of the right subdural hematoma. Preoperative coagulation parameters (prothrombin time, activated partial thromboplastin time, fibrinogen, thrombin time, and platelet count) were normal. The patient was too dysphoric to undergo surgery owing to increasing headache and confusion. Surgery was performed under general anesthesia with slight head rotation. Frontal and parietal burr-holes were drilled, followed by continuous subdural irrigation with tepid isotonic saline. The operation lasted 65 min. Perioperative blood pressure remained within the normal range. The drainage tube was connected to a closed collection system, which was fixed to the bed at the level of the patient's head. The patient's Glasgow Coma Score (GCS) improved from 12 to 15 postoperatively. A total of 220 mL, 190 mL, and 90 mL of cerebrospinal fluid (CSF) were drained from the subdural space at 24 h, 48 h, and 60 h, respectively. The patient suddenly developed nausea and vomiting, followed by deterioration of consciousness and stupor, 60 h after the evacuation. An urgent CT scan was performed, which demonstrated a significant hematoma in bilateral cerebellar hemispheres (diameter >4 cm) and a small hematoma in the frontal lobe (Figures 2a and 2b). At that time, the coagulation parameters in the blood were normal. In view of the patient's concurrent medical problems, with worsening respiratory function and poor prognosis, he was not deemed suitable for posterior fossa surgery. The patient was administered hyperosmolar therapy (250 mL of 20% mannitol every 6 h, 100 mL of 20% albumin per day), infection prophylaxis, and bed rest. The patient's GCS which was maintained at 11–12, gradually improved to 15 within 3 weeks. Repeat CT scans, which were performed 4, 7, 11, and 19 days after the operation, revealed that the bilateral cerebellar hemorrhage and frontal lobe hemorrhage had been largely absorbed (Figures 2c–2j). However, considering he had stent implantation history, it was risky to perform magnetic resonance imaging (MRI). Therefore, a preoperative CT angiography (CTA) was performed. CTA and CT venography revealed a hypoplastic right vertebral artery, and revealed dominant transverse sinus, mainly on the right side. The left side of intracranial segment of internal jugular vein and sigmoid sinus was totally undeveloped (Figure 3). The patient gradually recovered. He was discharged one month after surgery, without neurological complaints or deficits on examination.
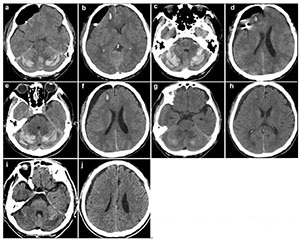
|
| Figure 2 Computed tomography images obtained after surgery (a, b: 60 hours; c, d: 4 days; e, f: 7 days; g, h: 11 days; i, j: 19 days). |
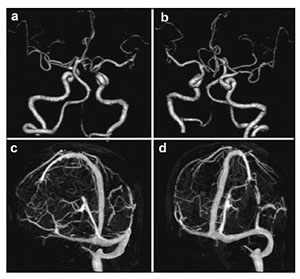
|
| Figure 3 Preoperative computed tomography angiography (CTA; a, b) and computed tomography venography (CTV; c, d) of the brain of the patient. |
In the current case, hematoma occurred near the surgical site and remote cerebellar region. We considered that the small frontal hematoma was of arterial origin caused by brain tissue stretching. CT appearances of the postoperative RCH suggested a venous origin. Contrary to arterial hypertensive bleeding, these hemorrhages were located almost uniformly symmetrically in the upper folia of the cerebellum. As previously stated[4], they were diffuse and vaguely circumscribed in the transverse oriented.
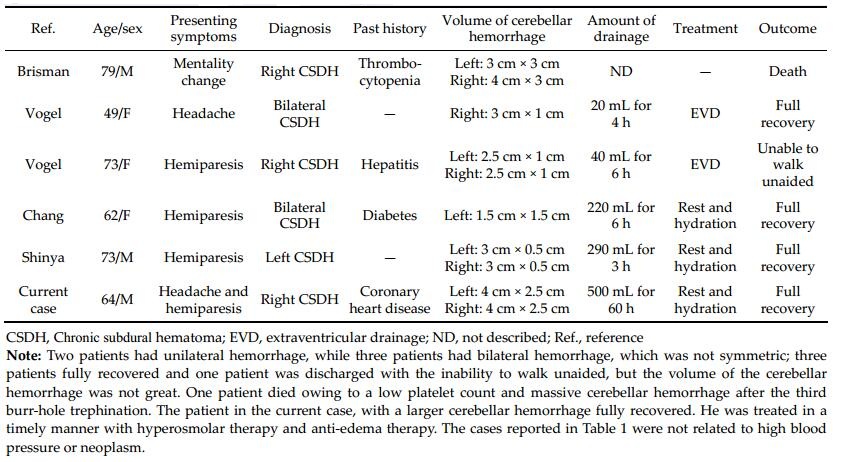
We performed a PubMed search and found five cases of RCH after burr-hole evacuation for CSDH[5–8]. The characteristics of our case and the five other cases are summarized in Table 1.
|
The precise pathomechanism for RCH following supratentorial craniotomy is unknown. Commonly reported factors for RCHs include arterial hypertension, anticoagulant treatment, blood dyscrasia, or coagulopathy[9]. In the current case, anti-platelet therapy was used until one month prior to the surgery, but the patient's coagulation parameters were normal in the perioperative period.
Brain retraction and excessive CSF loss during surgery may be important in the pathophysiological development of RCH. It shifts the intracranial contents with resultant stretching, compression, or an increase in the transmural pressure of the cerebellar draining veins or cerebellar parenchymal vessels[10]. Further, postoperative intradural or extradural drains may negatively lead to a suction effect on the brain and the cerebellum[2]. Moreover, the retrospective study conducted Toczek et al.[11] revealed that the CSF drainage apparatus was installed postoperatively in all patients with RCH after supratentorial surgery. A relatively large amount of fluid was drained from our patient as well.
However, CSF loss alone does not cause RCH. Many neurosurgical centers have routinely performed extensive opening of the cisterns without observing an increase in the occurrence of RCH. The positioning of the head during surgery of the patient should also be considered in this case. Excessive head rotation coupled with hyperextension can lead to obstruction of the jugular vein[12]. Transverse sinuses that are of unequal size, which do not communicate at the torcula, may easily result in venous infarction with hemorrhage in patients[13]. Our patient had a totally undeveloped left intracranial segment of the internal jugular vein. The sigmoid sinus was at a greater risk of suffering occlusion of the cerebellar venous system when draining veins on the other side was compressed owing to head rotation. Quick clearance of the subdural hematoma coupled with the drainage of the excessive CSF could have rapidly reduced the supratentorial pressure. An increase in the transmural pressure between the supratentorial and subtentorial region could have subsequently shifted the cerebellum, possibly resulting in damage to the cerebellar draining veins, which in turn may have led to cerebellar hemorrhage.
Until now, there has been no clear consensus on the indication of surgical treatment of cerebellar hemorrhage. Many scholars suggest that large hematomas (>3 cm) should be managed surgically. However, the patient in the current study had large bilateral hematomas (>4 cm) and a lower GCS (11), but made full recovery with conservative management. Therefore, we believe that surgical treatment may not be necessary in elderly patients with a large venous hematoma because when compared to arterial hematomas, venous hematomas are diffuse and its space-occupying effect is not obvious.
4 ConclusionsThe mechanism of cerebellar hemorrhage in burrhole evacuation for supratentorial chronic subdural hematoma is unknown. According to the analysis of this case, intracranial hypotension through excessive CSF loss and increase in the transmural pressure of cerebellar draining veins leading to vein injury may be the mechanism of remote cerebellar hemorrhage. This case has some significance in revealing the mechanism of remote cerebellar hemorrhage occurring after other supratentorial surgery.
Conflict of interests
All contributing authors have no conflict of interests. The authors declare that they do not have professional or financial affiliations that may have biased the results.
| [1] | Brisman MH, Bederson JB, Sen CN, Germano IM, Moore F, Post KD. Intracerebral hemorrhage occurring remote from the craniotomy site. Neurosurgery, 1996, 39(6): 1114–1121. DOI:10.1097/00006123-199612000-00009 |
| [2] | Honegger J, Zentner J, Spreer J, Carmona H, SchulzeBonhage A. Cerebellar hemorrhage arising prospectively as a complication of supratentorial surgery:A retrospective study. J Neurosurg, 2002, 96(2): 248–254. DOI:10.3171/jns.2002.96.2.0248 |
| [3] | Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases:Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo), 2001, 41(8): 371–381. DOI:10.2176/nmc.41.371 |
| [4] | Kalfas IH, Little JR. Postoperative hemorrhage:A survey of 4992 intracranial procedures. Neurosurgery, 1988, 23(3): 343–347. DOI:10.1227/00006123-198809000-00010 |
| [5] | Hyam JA, Turner J, Peterson D. Cerebellar haemorrhage after repeated burr hole evacuation for chronic subdural haematoma. J Clin Neurosci, 2007, 14(1): 83–86. DOI:10.1016/j.jocn.2005.12.048 |
| [6] | Vogels RLC, Verstegen MJT, van Furth WR. Cerebellar haemorrhage after non-traumatic evacuation of supratentorial chronic subdural haematoma:Report of two cases. Acta Neurochir (Wien), 2006, 148(9): 993–996. DOI:10.1007/s00701-006-0800-y |
| [7] | Chang SH, Yang SH, Son BC, Lee SW. Cerebellar hemorrhage after burr hole drainage of supratentorial chronic subdural hematoma. J Korean Neurosurg Soc, 2009, 46(6): 592–595. DOI:10.3340/jkns.2009.46.6.592 |
| [8] | Kobayashi S, Mutoh T, Ishikawa T, Moroi J, Tamagawa N, Yoshioka S, Hikichi K, Suzuki A. Remote cerebellar hemorrhage after single burr hole drainage of chronic subdural hematoma of the elderly. No Shinkei Geka, 2011, 39(8): 755–761. |
| [9] | Yasargil MG, Yonekawa Y. Results of microsurgical extraintracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery, 1977, 1(1): 22–24. DOI:10.1227/00006123-197707000-00005 |
| [10] | Park JS, Hwang JH, Park J, Hamm IS, Park YM. Remote cerebellar hemorrhage complicated after supratentorial surgery:Retrospective study with review of articles. J Korean Neurosurg Soc, 2009, 46(2): 136–143. DOI:10.3340/jkns.2009.46.2.136 |
| [11] | Toczek MT, Morrell MJ, Silverberg GA, Lowe GM. Cerebellar hemorrhage complicating temporal lobectomy. Report of four cases. J Neurosurg, 1996, 85(4): 718–722. DOI:10.3171/jns.1996.85.4.0718 |
| [12] | Seoane E, Rhoton AL Jr. Compression of the internal jugular vein by the transverse process of the atlas as the cause of cerebellar hemorrhage after supratentorial craniotomy. Surg Neurol, 1999, 51(5): 500–505. DOI:10.1016/S0090-3019(97)00476-X |
| [13] | Cohen ZR, Ram Z, Knoller N, Peles E, Hadani M. Management and outcome of non-traumatic cerebellar haemorrhage. Cerebrovasc Dis, 2002, 14(3-4): 207–213. DOI:10.1159/000065666 |



