2. Department of Neurosurgery, Tsinghua University Yuquan Hospital, Beijing 100040, China;
3. School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China
Lin Chen got MD under the tutelage of Professor Zonghui Liu from Department of Neurosurgery, Navy General Hospital of PLA and fellowship under the tutelage of Professor Huancong Zuo of Department of Neurosurgery, Tsinghua University Yuquan Hospital. Dr. Chen is an expert in treating trigeminal neuralgia via microvascular decompression surgery. Dr. Chen is the member of the medical ethics committee of Tsinghua University, secretary general of the International Society of Nerve Repair, standing member and deputy director general of the Chinese Medical Doctor Association Neural Restoration Specialized Committee.
Zhiye Qiu received his PhD degree from Huazhong University of Science and Technology, worded as a postdoctoral fellow at School of Materials Science and Engineering, Tsinghua University, and is now working as vice-general manager of Beijing Allgens Medical Science and Technology Co., Ltd. His research interests are focused on biomimetic artificial collagen/hydroxyapatite composites and their clinical applications on bone defect repair. Dr. Qiu is currently in charge of several R&D projects of new bone graft products and some governmental scientific research funds on biomedical materials.
Yuqi Zhang is a Ph.D. Tutor, a well-known expert in neurosurgery, and a leading pediatric neurosurgeon in the Chinese Community. He is the Vice President of Tsinghua University’s Medical School and President of Tsinghua University’s Second Affiliated Hospital. He studied neurosurgery in the United States as post-doctoral researcher, and was for twelve years the Director of Pediatric Neurosurgery at Beijing Tiantan Hospital. He also served as the Chinese Journal of Neurosurgery’s Editorial Director for nine years. He is a council member of the Chinese Physicians’ Association, the Vice Chairman of the Neurological Surgeons’ Chapter of the Chinese Medical Association, a Chinese member of the International Society for Pediatric Neurosurgery (ISPN), and an editorial board member of several distinguished journals, including the Chinese Journal of Neurosurgery and the Journal of Pediatric Surgery.
Tianxi Song is Manager of Technical Department, Master of Polymer Material Science of Dalian University of Technology (DUT). Research Interests: D&R of Medical devices. Publication includes more than 10 SCI papers.
Fuzhai Cui is Biomaterials Professor of Tsinghua University and Fellow of American Institute of Medical and Biological Engineering (2007). Research Interests: Biomaterials for bone and nerve repair. Publication includes more then 400 SCI papers, H factor is 57.
Bony defects in the craniomaxillofacial skeleton remain a major and challenging health concern. Repairing the cranial bone is one of the oldest neurosurgical practices dating back to ancient Egypt. Rehabilitating the natural contours of the skull has been challenging the most ingenious surgeons from antiquity to nowadays. Given the persistent progress in the neurosurgical and first-aid treatment during the past centennial practice, more patients have survived such head injuries, thus simple, safe, and durable means are needed to correct skull defects more than ever before. In response, countless materials and techniques have been devised along with the improving art of cranioplasty. After centuries of evolution, the objective of cranioplasty that is to restore the outermost part of neurocranium has remained unchanged. Numerous materials have been developed to repair bone defect in the skull, including autograft, allograft, as well as artificial or synthetic biomaterials[1-3]. Thanks to the evolution of material sciences and novel techniques, some of the once aggrandized materials have been eliminated from the selections because of noted side effects. Cranial bone defects may be either congenital, such as nosencephalia and dysraphism, or acquired, like trauma. It could even be iatrogenic caused by maxillofacial or encephalic surgeries. Cranial bone defects are always accompanied by critical complications, including epilepsy, cerebritis, encephalitis, hydrocephalus, and psychological or mental disorders[4, 5]. Obviously, meeting both cosmetic and functional demands, effective cranioplasty plays a pivotal role to maintain ideal cerebral protection and consummate cosmetic result. Especially for pediatric patients, expeditious and effective cranioplasty to synchronize with growing skull of child is of great concern[6, 7].
In surgical intervention, biomaterials are widely employed to repair skull defects or deformity.
Cranioplasty can be performed using multifarious materials ranging from autogeneic tissue to metallic or acrylic alloplastic implants. Besides natural materials such as autograft, allograft, or xenograft, varieties of artificial materials such as titanium, poly (methyl methacrylate) (PMMA), polyether ether ketone (PEEK), and hydroxylapatite (HA) have been developed. Although partly meeting certain requirements such as mechanical strength and biocompatibility, none of these artificial substitutes is perfect. In the modern era, despite traffic accidents and military conflicts, people have got greater survivorship after cranial injury than in the past. People attribute this triumph to improved medical practice and early "far-forward" neurosurgical treatment. The last decade has seen breathtaking advancement in cranioplasty and cranioprostheses. Custom-made alloplast implants and regenerative cranial treatments have emerged. Medical techniques such as protected bone regeneration, free tissue transfers, and distraction osteogenesis have found their niches in skull renovation. In addition, comprehensive rehabilitation has been newly acknowledged as a standard treatment in case of neurotrauma.
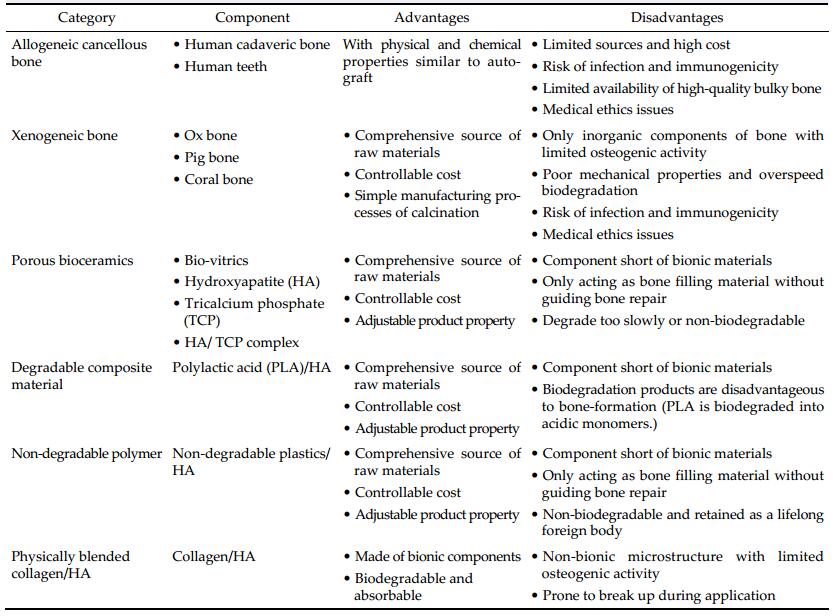
2 Traditional skull repair materials 2.1 Biological materialsPreeminent in osteoconductivity, osteoinductivity, biocompatibility, biodegradability, and free of immunologic rejection, autogeneic bone has incontrovertibly been widely recognized as the "gold standard" for healing large-sized bone defects. Autogeneic bones could be derived from many parts of human body, such as ilium, fibula, rib, as well as debris collected during craniotomy. For example, in case of burr-holes or small defects created in the skull, surgeons usually collect bone debris produced by trepanation and glue them back. Moreover, cortical bone discs gleaned from the inner osseous lamella could be used for filling defects in the outer lamella. Ribs and fibula are common source of autograft. A 30-year follow-up proved that rib grafts could renovate skull defects both cosmetically and functionally and even accommodate itself to the growth of puerile skeleton of children[8].
Despite these advantages, many serious limitations that are associated with autogeneic implant cannot inevitably be overlooked. It is hard to shape autogeneic bone to match the contour of the defected skull. Besides, it inevitably imposes morbidities at the donor site, such as thorax deformities and respiratory problems[2]. Moreover, availability of autograft is highly limited, especially for patients suffering from systemic complications.
The use of allogenous grafts in cranioplasty also has more than a century long history. Allogeneic cartilage was firstly introduced to cranioplasty during the First World War and has been popularized ever since. However, cartilage transplantation fell into disfavor before long due to the insufficiency in calcification and mechanical strength. During the same period, cadaveric skull bone was also used as grafts in cranioplasty[9]. After harvested from corpse, the unwanted ingredients within cadaveric allografts were disposed with chemical reagents and heat sterilization. However, probability of infection and excessive absorption are intractable problems of allografts. Those lethal disadvantages, let alone ineluctable ethical issues which exacerbate its inferiority, limit its clinical generalization. In brief, compared to autoplasts and synthetic materials, allografts have been much more disfavored and ignored.
2.2 Metallic materialsTantalum used to be the most popular artificial substitute of allograft for cranioplasty because of its biochemical inertia which makes it resistant to acid, oxidation, and even corrosion. The Second World War greatly increased the demand for cranioplasty procedures and tantalum cranioprostheses hence became popular amongst military surgeons[10]. Tantalum represented a significant breakthrough in synthetic cranioplasty and renewed interest in the search for a suitable synthetic material for cranioprostheses. The experiences of wartime neurosurgeons with tantalum cranioplasty played a pivotal role in the evolution of modern cranioplasty techniques and ultimately led to a better understanding of the necessary attributes of an ideal synthetic cranioprostheses[11]. As it has the ability to be easily cut and shaped, tantalum meets the requirements for repairing a considerable part of cranial defects, especially those resulting from war wounds. On the other hand, tantalum is a heat conductor, thus leading to sharp sense of temperature for the patients and potentially harming the brain. Moreover, tantalum imposes significant image artifacts upon computed tomography (CT) and magnetic resonance imaging (MRI) besides being too expensive to be commercialized at a large scale. Despite all these flaws, the history of tantalum cranioplasty serves as a model for innovative thinking and adaptive technology development.
Usually used in the form of mesh, titanium has become the most popular metallic cranioprostheses due to its good quality in biocompatibility, radiolucency, mechanical strength, and density. With the development of computer aided design and computer aided manufacturing (CAD/CAM), custom-fabricated titanium cranioprostheses have been commercially available for restoring irregular skull defects or deformities[12]. Despite all these virtues, there have been studies highlighting that cranioplasty is associated with significant complications including infections, seromas, and hematomas with incidence as high as up to 30%[13]. Some of the complications are so severe that patients usually need reoperation to remove implant[14]. All these studies have provided better understanding of the risks associated with titanium cranioplasty and contribute to decision making by the clinicians and patients. To overcome these shortcomings, scientists discovered titanium fiber mesh discs coated with a thin layer of hydroxyapatite (HA). In vivo experiments revealed that the thin layer of HA coating could significantly enhance osteogenetic activity and regeneration of osseous tissues in the titanium fiber mesh via acting as a bone regeneration scaffold[15].
2.3 Ceramic materialsDue to satisfactory physicochemical properties, mechanical strength, and biocompatibility, alumina bioceramics have been widely used as implants in stomatology and cranioplasty. However, congenital brittleness, inflexibility, and deficient plasticity hinder their access to widespread application.
Calcium phosphate is chemically similar to the inorganic component of natural bone and has outstanding biocompatibility and osteoconductivity analogous to autoplast[16]. Calcium phosphate is therefore used as an active ingredient in bone cement, acting as an adhesive in cranioplasty. However, calcium phosphate must be modified to improve its remedial effect because it is so fragile as to be easily fractured even due to minor impact[17].
Formulated as Ca10(PO4)6(OH)2, HA is the major inorganic component of bones and is gifted with inherent biocompatibility. Therefore, HA ceramics have been widely used for repairing bone defect in cranioplasty[18]. Custom-fabricated cranioprostheses made of HA were also developed in recent years[19, 20]. However, the merits of HA ceramics are also drawn back by its brittleness.
2.4 Polymeric materialsNeurosurgeons sometimes perform revision cranioplasty in patients with titanium allergy using prefabricated, custom-made poly (methyl methacrylate) (PMMA) implant with modified fixation technique. Among acrylic materials, PMMA is by far the material most frequently implanted to substitute bones in vertebroplasty[21, 22]. After long-time follow-ups of randomized, blinded, parallel controlled and multiple center trial, safety and efficacy of PMMA prostheses in clinical treatment of large skull defect have been fully approved[23]. However, segments augmented using PMMA within the osteoporotic spine may also be related to increased risk of fracture of the adjacent vertebral bodies because it has significantly higher modulus of elasticity than normal osseous tissue. Fracture is a common sequel of osteoporotic spine especially when PMMA is applied as a filling material in transpedicular balloon kyphoplasty for spinal compression fractures[24]. When PMMA is used as the major active ingredient of bone cement in vertebroplasty, there are serious complications such as cement leakage. PMMA bone cement could also harm surrounding tissues by releasing heat and potentially neurotoxic monomer during polymerization, or even being chemotoxic to dura mater and cerebral cortex[25].
Polyether ether ketone (PEEK) is another kind of popular polymeric materials for skull renovation. With excellent performance in nature, PEEK has been popularized just a decade ago[26]. Outstanding in aspect of density, mechanical strength, and elastic modulus, PEEK is applied to omnibearing process of cranioplasty. In addition to functionally comparable to autoplast, PEEK does not interfere with CT or MRI alike. Breathtakingly, PEEK implants can be easily designed and fabricated according to custom order[27, 28]. Despite all the investment in clinical development in the present, the insufficient osteointegration and exorbitant cost of this technique impede its further commercial dissemination[3]. Alternatively, although PEEK lacks osteoconductive properties, as an inert and easily removable material, it has proven useful in settings where repeated intracranial access is necessary.
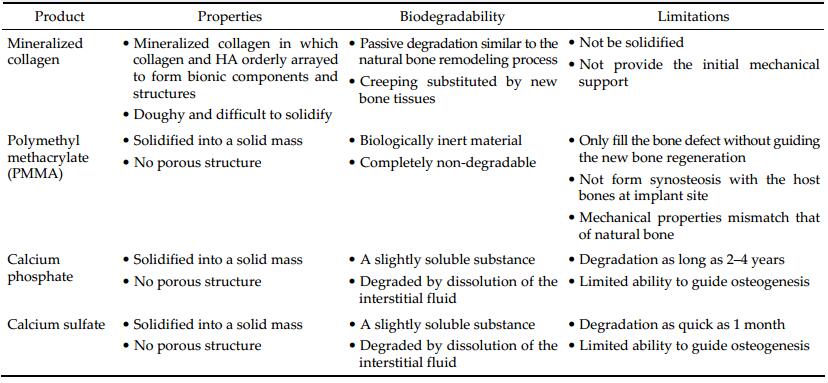
3 Mineralized collagenDespite the ground rapidly gained in neurosurgical operations, most of these prevalent synthetic bone substitutes are not susceptible to biodegradation. In most cases of pediatric patients whose skulls are undergoing continual growing and renewal, prostheses made of non-degradable materials even result in deformities of the skull[7]. Therefore, those characteristically refractory materials are not feasible for pediatric surgery and this opinion is defended by both neurosurgeons and material scientists. There is still a comprehensive controversy on the timing of pediatric neurosurgery[29, 30]. Not able to actively participating in osteogenesis and bone absorption, those fabricated cranioplasty materials applied in the moment only reconstruct the skull contour without physiologically restoring it. Skull repair materials are required to have mechanical and dynamical performance to similar osseous tissues. For example, PEEK (undertaking mechanics) cages have been successfully used with HA (dynamics) for anterior cervical discectomy with fusion. An ideal cranioprostheses must also be moldable or machinable to fit the shape of the skull defect. It should be osteoconductive and biodegradable to promote complete cicatrization. Besides, it should be ideally endowed with additional properties like radiolucency, anti-infection, and poor thermal conductivity[31].
Mineralized collagen (MC) builds the understructure for various connective tissues ranging from bone to dentin[32, 33]. It provides the calcified tissue structures with essential 3-dimensional structure and microecological niche for the adherence, proliferation and ossification effect of osteoblasts. Within the MC, HA crystals are orderly juxtaposed along fibrils fabricated by type Ⅰ collagen in a specific hierarchically staggered nanostructure. Foundation for the trivial physiological functions of compact bones and trabeculae is formed by MC. Two thirds of the dry weight in the bone matrices correspond to mineral components in the form of nanometric crystals and one third is formed by collagen fibrils[34]. The rationale for the assembling process of MC has been explained by different hypotheses, such as self-assembly process and polymer-induced liquid-precursor process[35]. Guided by biothermodynamics and related principles, synthetic biomaterials mimicking natural structure of MC have been flourishing[36].
Many protocols have been innovated to prepare bionic materials copying the native composition and structure of MC[37]. Most of those measures betook themselves to the composite ingredient of MC that comprises collagen and HA, disregarding the native status of their microstructure[38]. Some synthetic MC materials have been successfully developed and transcended their predecessors[39]. Hierarchical intrafibrillar MC is achieved through a selective mineralization process in the collagenous gap. The associated topographical features directly correlate with their nanomechanical heterogeneities to accommodate a broad range of external loads. Moreover, this hierarchically staggered nanostructure provides an optimized microenvironment to improve bone regeneration by instructing host cells. Both in vitro and in vivo experiments have shown that the MC bone cement has excellent biocompatibility. Many activated host fibroblasts infiltrated or grew into the MC implant, along with prominent neovascularization or abundant newly formed capillary vessels[40]. The results implied that MC can be absorbed and transformed into the main component of osseous tissues in the process of new bone formation. In summary, MC should be very promising for bone reparation.
Other than traditional synthetic bone grafts that only act as structural supersedes without any functional bioactivity, the MC is not only biocompatible, biodegradable, and absorbable, but also osteoconductive and osteoinductive. Prostheses made of MC could undergo "creeping substitution", a process of osteogenesis and bone regeneration after implantation. Nonetheless, concerning the short run effect, all prostheses should provide initial protection to the bone defect shortly after implantation. With less mechanical strength than metal materials, MC was considered inferior to metal materials regarding the aspect of providing initial stability before forming coalescent or confluent linkage with surrounding osseous tissues. This blind side of MC needs to be ameliorated by biomedical engineering. A comparison of the abovementioned major bone substitute materials is summarized in Table 1.
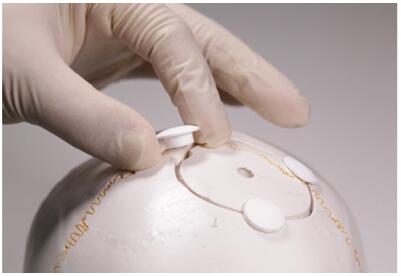
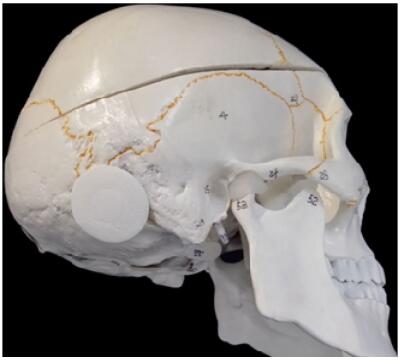
SkuhealTM is registered for a cavalcade of bone substitutes patented by Beijing Allgens Medical Science and Technology Co., Ltd. It is featured by a series of MC materials employed to repair cranial defects. SkuhealTM was developed based on breakthrough discoveries in MC biomaterials. It is the best-in-class bone graft for neurosurgeries. Its efficacy when mixing with bone marrow is as good as autograft. The SkuhealTM bone material imitates the composition and the micro-structure characteristics of natural bones. It has the ability of bonding with bones and it can be absorbed and translated to the main composition of a new bone. The materials have been approved, registered, and certificated by the China Food and Drug Administration (CFDA, 20143462075) and also patented by the United States of America[41]. Custommade SkuhealTM is easy to be used as repair plug in craniotomy (Figure 1) and trigeminal neuralgia decompression surgery (Figure 2). SkuhealTM burr-hole plug is a featured product that facilitates the reconstruction of burr-holes created by trephination and trepanation during cranioplasty, as well as small cranial defects produced by craniotomy such as those used in microvascular decompression. Like biocompatibility, bioresorbability, and osteoconductivity, MC materials have exactly the same advantageous characters as autologous bones. With the degradation of the graft made of MC, the temporarily installed substitute is susceptible to be gradually replaced by a new bone, in a biological process nominated as "creeping substitution".
|
| Figure 1 Custom-made prosthesis and skull repair plug applied in craniotomy. |
|
| Figure 2 Skull repair plug used to patch burr hole trepanned in trigeminal neuralgia decompression surgery. |
Allgens medical also offers series of products of bone substitute ranging from cylindrical (BonfillTM) to an injectable, malleable form (BonflowTM). All their active ingredients were MC with efficacy as good as autograft if mixing with autologous bone marrow. BongoldTM cost more than allogenous bone, but it has much lower risk of transmitted diseases. In turn, BongoldTM is more affordable than parallel artificial materials like PEEK. As far as bone cements, paste or putty is concerned, BonflowTM has very competitive price/performance ratio, which offers better curative effect and eases the burdens from patients. For neurosurgeons, it is easy to manipulate with simple operating procedures. It will not affect the practicing habit of neurosurgeons or prolong the surgical duration. A comparison of MC and traditional bone cements used in cranioplasty is summarized in Table 2.
Undergoing intramembranous rather than endochondral ossification, regeneration of cranial bone gives rise to a great challenge, as the renovation rates of flat bones like calvarium and ilium are much slower than those of long bones. This phenomenon is partly attributed to their distinguishing ossification processes. As an emblematic of flat bones, previous reports have shown that three principal pathways are involved in cranial bone regeneration. One is new bone ingrowth from the diploe, which is the spongy bone located in the intermediate layer of the skulls. The other is intramembranous ossification launched by the periosteum located on the outer surface of the cranial bones. It can trigger quiescent lining cells to differentiate into osteoblasts and hence deposit osteoid in the defect. Alternatively, osteogenesis could also originate from the outmost lamina of the dura mater. Because the periosteum is usually wrecked in the case of a cranial injury, the repair material should ideally be able to promote new bone generated in the diploe and/or from the dura mater. Additionally, the material should present exceptional mechanical performance to provide enough protection and keep a stable intracranial environment.
Obviously, cranioplasty aiming to repair cranial defect involves combinatorial aspects of surgical techniques and material sciences. For decades, professionals have been devoted themselves to improve the mechanical, dynamical, and biochemical qualities of bone substitutes. Nowadays, osteoinductivity is emphasized by surgeons and concerning specialists. Bioactive materials thereby gradually take the center stage. Many kinds of cytokines have been applied in clinical practice to increase biological activities of prostheses. For example, bone morphogenetic protein-2 (BMP-2) plays an important role in osteogenesis by involving itself in many important signaling pathways[42]. It potently induces manifold types of cells that differentiate into osteoblast and stimulate the regeneration of defected bone[43]. Implantation of BMP-2 is performed primarily in orthopedic procedures such as spinal interbody fusion cage, which uses a variety of materials including metals, ceramics, polymers, and composites[44]. Delivered via absorbable sponge, BMP-2 has been reported to successfully obtain and maintain intervertebral fusion. BMP-2 can improve clinical outcomes, and reduce pain after anterior lumbar interbody arthrodesis in patients with degenerative lumbar intervertebral disc disorders[45]. However, complications and adverse outcomes have been increasingly reported under administration of recombinant human BMP-2. In most cases, those untoward effects caused by BMP-2, such as pleural effusion, airway stenosis, and neurothlipsis (nerve compression), were reported in anterior cervical discectomy with fusion[46, 47]. Platelet-rich plasma (PRP) contains many nutritious cytokines and can be used in tissue rehabilitate. In combination with allogeneic bone marrow mesenchymal stem cells (BMSCs) or amniotic fluid-derived stem cells (AFSCs), PRP significantly promotes healing of osteoporotic bone defects in animal model[48, 49]. Other studies also provide in-depth knowledge regarding the use of stem cells and PRP in vitro and in vivo, and address their application in clinical studies in the future[50].
Facing the high paucity of bone grafts worldwide, regenerating bone is of prime concern due to the current demand of bone grafts and the increasing number of diseases causing bone loss. Many alternative strategies have emerged for the treatment of bone defect. Taken together, this review indicates the superiority of MC to other bone substitutes. Furthermore, it is feasible to compound bone with various bioactive materials including cytokines, plasma, and stem cells to restore bone defects.
Conflict of interests
All contributing authors have no conflict of interests as far as this review is concerned.
| [1] | Spetzger U, Vougioukas V, Schipper J. Materials and techniques for osseous skull reconstruction. Minim Invasive Ther Allied Technol, 2010, 19(2): 110–121. DOI:10.3109/13645701003644087 |
| [2] | Aydin S, Kucukyuruk B, Abuzayed B, Aydin S, Sanus GZ. Cranioplasty: Review of materials and techniques. J Neurosci Rural Pract, 2011, 2(2): 162–167. DOI:10.4103/0976-3147.83584 |
| [3] | Shah A M, Jung H, Skirboll S. Materials used in cranioplasty: A history and analysis. Neurosurg Focus, 2014, 36(4): E19. DOI:10.3171/2014.2.FOCUS13561 |
| [4] | Honeybul S, Ho KM. Long-term complications of decompressive craniectomy for head injury. J Neurotraum, 2011, 28(6): 929–935. DOI:10.1089/neu.2010.1612 |
| [5] | Chibbaro S, Di Rocco F, Mirone G, Fricia M, Makiese O, Di Emidio P, Romano A, Vicaut E, Menichelli A, Reiss A, et al. Decompressive craniectomy and early cranioplasty for the management of severe head injury: A prospective multicenter study on 147 patients. World Neurosurg, 2011, 75(3-4): 558–562. DOI:10.1016/j.wneu.2010.10.020 |
| [6] | Szpalski C, Barr J, Wetterau M, Saadeh PB, Warren SM. Cranial bone defects: Current and future strategies. Neurosurg Focus, 2010, 29(6): E8. DOI:10.3171/2010.9.FOCUS10201 |
| [7] | Khader BA, Towler MR. Materials and techniques used in cranioplasty fixation: A review. Mater Sci Eng C Mater Biol Appl, 2016, 66: 315–322. DOI:10.1016/j.msec.2016.04.101 |
| [8] | Beekmans SJ, Don Griot JP, Mulder JW. Split rib cranioplasty for aplasia cutis congenita and traumatic skull defects: More than 30 years of follow-up. J Craniofac Surg, 2007, 18(3): 594–597. DOI:10.1097/scs.0b013e3180576f44 |
| [9] | Feroze AH, Walmsley GG, Choudhri O, Lorenz HP, Grant GA, Edwards MSB. Evolution of cranioplasty techniques in neurosurgery: Historical review, pediatric considerations, and current trends. J Neurosurg, 2015, 123(4): 1098–1107. DOI:10.3171/2014.11.JNS14622 |
| [10] | Flanigan P, Kshettry VR, Benzel EC. World War Ⅱ, tantalum, and the evolution of modern cranioplasty technique. Neurosurg Focus, 2014, 36(4): E22. DOI:10.3171/2014.2.FOCUS13552 |
| [11] | Bonfield CM, Kumar AR, Gerszten PC. The history of military cranioplasty. Neurosurg Focus, 2014, 36(4): E18. DOI:10.3171/2014.1.FOCUS13504 |
| [12] | Sunderland IRP, Edwards G, Mainprize J, Antonyshyn O. A technique for intraoperative creation of patient-specific titanium mesh implants. Plast Surg (Oakv), 2015, 23(2): 95–99. |
| [13] | Mukherjee S, Thakur B, Haq I, Hettige S, Martin AJ. Complications of titanium cranioplasty: A retrospective analysis of 174 patients. Acta Neurochirurg, 2014, 156(5): 989–998. DOI:10.1007/s00701-014-2024-x |
| [14] | Williams LR, Fan KF, Bentley RP. Custom-made titanium cranioplasty: Early and late complications of 151 cranioplasties and review of the literature. Int J Oral Maxillofac Surg, 2015, 44(5): 599–608. DOI:10.1016/j.ijom.2014.09.006 |
| [15] | Hirota M, Hayakawa T, Yoshinari M, Ametani A, Shima T, Monden Y, Ozawa T, Sato M, Koyama C, Tamai N, et al. Hydroxyapatite coating for titanium fibre mesh scaffold enhances osteoblast activity and bone tissue formation. Int J Oral Maxillofac Surg, 2012, 41(10): 1304–1309. DOI:10.1016/j.ijom.2011.12.035 |
| [16] | Kuemmerle JM, Oberle A, Oechslin C, Bohner M, Frei C, Boecken I, von Rechenberg B. Assessment of the suitability of a new brushite calcium phosphate cement for cranioplasty— An experimental study in sheep. J Craniomaxillofac Surg, 2005, 33(1): 37–44. DOI:10.1016/j.jcms.2004.09.002 |
| [17] | Matic DB, Manson PN. Biomechanical analysis of hydroxyapatite cement cranioplasty. J Craniofac Surg, 2004, 15(3): 415–422. DOI:10.1097/00001665-200405000-00012 |
| [18] | Shirai T, Tsuchiya H, Terauchi R, Tsuchida S, Mizoshiri N, Ikoma K, Fujiwara H, Miwa S, Kimura H, Takeuchi A, et al. Treatment of a simple bone cyst using a cannulated hydroxyapatite pin. Medicine, 2015, 94(25): e1027. DOI:10.1097/MD.0000000000001027 |
| [19] | Staffa G, Barbanera A, Faiola A, Fricia M, Limoni P, Mottaran R, Zanotti B, Stefini R. Custom made bioceramic implants in complex and large cranial reconstruction: A twoyear follow-up. J Craniomaxillofac Surg, 2012, 40(3): e65–e70. DOI:10.1016/j.jcms.2011.04.014 |
| [20] | Stefini R, Zanotti B, Nataloni A, Martinetti R, Scafuto M, Colasurdo M, Tampieri A. The efficacy of custom-made porous hydroxyapatite prostheses for cranioplasty: Evaluation of postmarketing data on 2697 patients. J Appl Biomater Funct Mater, 2015, 13(2): e136–e144. |
| [21] | Fischer CM, Burkhardt JK, Sarnthein J, Bernays RL, Bozinov O. Aesthetic outcome in patients after polymethylmethacrylate (PMMA) cranioplasty—A questionnaire-based single-centre study. Neurol Res, 2012, 34(3): 281–285. DOI:10.1179/1743132812Y.0000000007 |
| [22] | Caro-Osorio E, De la Garza-Ramos R, Martínez-Sánchez SR, Olazarán-Salinas F. Cranioplasty with polymethylmethacrylate prostheses fabricated by hand using original bone flaps: Technical note and surgical outcomes. Surg Neurol Int, 2013, 4: 136. |
| [23] | Jaberi J, Gambrell K, Tiwana P, Madden C, Finn R. Longterm clinical outcome analysis of poly-methyl-methacrylate cranioplasty for large skull defects. J Oral Maxillofac Surg, 2013, 71(2): e81–e88. DOI:10.1016/j.joms.2012.09.023 |
| [24] | Quan RF, Ni YM, Zhang L, Xu JW, Zheng X, Yang DS. Short-and long-term effects of vertebroplastic bone cement on cancellous bone. J Mech Behav Biomed Mater, 2014, 35: 102–110. DOI:10.1016/j.jmbbm.2014.03.007 |
| [25] | Pikis S, Goldstein J, Spektor S. Potential neurotoxic effects of polymethylmethacrylate during cranioplasty. J Clin Neurosci, 2015, 22(1): 139–143. DOI:10.1016/j.jocn.2014.06.006 |
| [26] | Rosenthal G, Ng I, Moscovici S, Lee KK, Lay T, Martin C, Manley GT. Polyetheretherketone implants for the repair of large cranial defects: A 3-center experience. Neurosurgery, 2014, 75(5): 523–529. DOI:10.1227/NEU.0000000000000477 |
| [27] | Rammos CK, Cayci C, Castro-Garcia JA, Feiz-Erfan I, Lettieri SC. Patient-specific polyetheretherketone implants for repair of craniofacial defects. J Craniofac Surg, 2015, 26(3): 631–633. DOI:10.1097/SCS.0000000000001413 |
| [28] | Alonso-Rodriguez E, Cebrian JL, Nieto MJ, Del Castillo JL, Hernández-Godoy J, Burgueño M. Polyetheretherketone custom-made implants for craniofacial defects: Report of 14 cases and review of the literature. J Craniomaxillofac Surg, 2015, 43(7): 1232–1238. DOI:10.1016/j.jcms.2015.04.028 |
| [29] | Waqas M, Ujjan B, Hadi YB, Najmuddin F, Laghari AA, Khalid S, Bari ME, Bhatti UF. Cranioplasty after craniectomy in a pediatric population: Single-center experience from a developing country. Pediatr Neurosurg, 2017, 52(2): 77–79. DOI:10.1159/000452808 |
| [30] | Zhang C, Wang Z, Shi R, Feng S, Sun J, Wang W, Su L, Zhang A. Feasibility study on repair for cranial defect of immaturity. Turk Neurosurg, 2013, 23(3): 355–358. |
| [31] | Mishra R, Goel SK, Gupta KC, Kumar A. Biocomposite cryogels as tissue-engineered biomaterials for regeneration of critical-sized cranial bone defects. Tissue Eng Part A, 2014, 20(3-4): 751–762. |
| [32] | Schwartz AG, Pasteris JD, Genin GM, Daulton TL, Thomopoulos S. Mineral distributions at the developing tendon enthesis. PLoS One, 2012, 7(11): e48630. DOI:10.1371/journal.pone.0048630 |
| [33] | Weisgerber DW, Erning K, Flanagan CL, Hollister SJ, Harley BAC. Evaluation of multi-scale mineralized collagenpolycaprolactone composites for bone tissue engineering. J Mech Behav Biomed Mater, 2016, 61: 318–327. DOI:10.1016/j.jmbbm.2016.03.032 |
| [34] | Zhou HJ, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater, 2011, 7(7): 2769–2781. DOI:10.1016/j.actbio.2011.03.019 |
| [35] | Landis WJ, Silver FH. Mineral deposition in the extracellular matrices of vertebrate tissues: Identification of possible apatite nucleation sites on type Ⅰ collagen. Cells Tissues Organs, 2009, 189(1-4): 20–24. |
| [36] | Ma J, Wang JL, Ai X, Zhang SM. Biomimetic self-assembly of apatite hybrid materials: From a single molecular template to bi-/multi-molecular templates. Biotechnoy Adv, 2014, 32(4): 744–760. DOI:10.1016/j.biotechadv.2013.10.014 |
| [37] | Qiu ZY, Tao CS, Cui HL, Wang CM, Cui FZ. High-strength mineralized collagen artificial bone. Front Mater Sci, 2014, 8(1): 53–62. DOI:10.1007/s11706-014-0237-9 |
| [38] | Lv YM, Yu QS. Repair of articular osteochondral defects of the knee joint using a composite lamellar scaffold. Bone Joint Res, 2015, 4(4): 56–64. DOI:10.1302/2046-3758.44.2000310 |
| [39] | Kou JM, Fu TY, Jia XJ, Hou JW, Gao C, Ma YZ, Qiu ZY, Cui FZ. Clinical observations on repair of non-infected bone nonunion by using mineralized collagen graft. J Biomater Tissue Eng, 2014, 4(12): 1107–1112. DOI:10.1166/jbt.2014.1258 |
| [40] | Liu X, Wang XM, Chen ZG, Cui FZ, Liu HY, Mao KY, Wang Y. Injectable bone cement based on mineralized collagen. J Biomed Mater Res B Appl Biomater, 2010, 94B(1): 72–79. |
| [41] | Cui FZ, Zhang SM, Zhang W, Cai Q, Feng QL. Nanocalcium phosphates/collagen based bone substitute materials. U.S. Patent 6, 887, 488 B2, May 3, 2005. |
| [42] | Iwasaki M, Nakahara H, Nakase T, Kimura T, Takaoka K, Caplan AI, Ono K. Bone morphogenetic protein 2 stimulates osteogenesis but does not affect chondrogenesis in osteochondrogenic differentiation of periosteum-derived cells. J Bone Miner Res, 1994, 9(8): 1195–1204. |
| [43] | Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev, 2003, 55(12): 1613–1629. DOI:10.1016/j.addr.2003.08.010 |
| [44] | Agrawal V, Sinha M. A review on carrier systems for bone morphogenetic protein-2. J Biomed Mater Res B Appl Biomater,, 2016, 104(1). |
| [45] | Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine, 2006, 31(7): 775–781. DOI:10.1097/01.brs.0000206357.88287.5a |
| [46] | Stiel N, Hissnauer TN, Rupprecht M, Babin K, Schlickewei CW, Rueger JM, Stuecker R, Spiro AS. Evaluation of complications associated with off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in pediatric orthopaedics. J Mater Sci Mater Med, 2016, 27(12): 184. DOI:10.1007/s10856-016-5800-8 |
| [47] | Poeran J, Opperer M, Rasul R, Mazumdar M, Girardi FP, Hughes AP, Memtsoudis SG, Vougioukas V. Change in off-label use of bone morphogenetic protein in spine surgery and associations with adverse outcome. Global Spine J, 2016, 6(7): 650–659. DOI:10.1055/s-0036-1571284 |
| [48] | Wei B, Huang CS, Zhao MY, Li P, Gao X, Kong JC, Niu YR, Huang R, Quan JH, Wei JS, et al. Effect of mesenchymal stem cells and platelet-rich plasma on the bone healing of ovariectomized rats. Stem Cells Int, 2016, 2016: 9458396. |
| [49] | Wang MJ, Li HL, Si JW, Dai JW, Shi J, Wang XD, Guo LH, Shen GF. Amniotic fluid-derived stem cells mixed with platelet rich plasma for restoration of rat alveolar bone defect. Acta Biochim Biophys Sin,, 2017, 49(3): 197–207. |
| [50] | Fernandes G, Yang SY. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res, 2016, 4: 16036. DOI:10.1038/boneres.2016.36 |