2. Aston Institute of Regenerative Medicine, Aston Medical School, Aston University, Birmingham, UK;
3. Molecular Pharmacology Laboratory, Institute of Molecular Medicine, Peking University, Beijing 100871, China;
4. Department of Psychology, Zhongda Hospital, Southeast University, Nanjing 210009, China
Brain ischemic stroke or cerebrovascular accident is one of the most common medical illnesses. Stroke always results in major changes in a person, such as losses in health, occupation, or independence. Major neuro-psychiatric (NP) disorders such as depression and apathy occur after stroke. In addition to the general or physio-therapeutics of the stroke survivor, the recogni-tion and treatment of NP disorders are also important. The NP disorders in stroke patients can heavily influ-ence disability, poor rehabilitation, and even mortality.
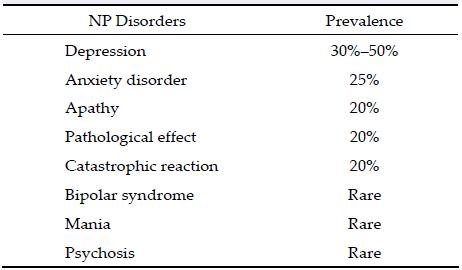
Post-stroke NP disorders are common and related with a complete spectrum of psychiatric illnesses. Depression, anxiety, emotional incontinence, and cata-strophic reactions are the most common manifestations of NP disorders.
In the current study, we reviewed post-stroke NP disorders that potentially affected the rehabilitation of stroke survivors. First, we reviewed the prevalence of post-stroke NP disorders and then focused on the etiology and management of post-stroke depression (PSD) as well as the characteristics of and biological treatments for post-stroke apathy, catastrophic reaction, pathological effect, psychosis, and mania.
Depression is a quite common problem in post-stroke patients that affects rehabilitation, recovery, and quality of life. The frequency of PSD is reportedly 10%-40%. Moreover, the reported prevalence of PSD varies over time from 20% to 50% in the first year and with a prominent peak within first 6 months after the stroke. Such a wide range of prevalence is due to variation in data capture methods and the following criteria:
(1) Use of different scales for the diagnosis ranging from self-rating scales to properly structured interview scales;
(2) The mean prevalence of patients examined in the hospitalized acute stroke patients was high (30%- 40%) compared to community samples (10%-20%);
(3) Duration-whether the patients were studied during the acute phase, chronic phase, or later in life after stroke.
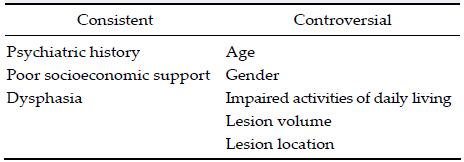
The risk factors for PSD are considered consistent and controversial.
The etiology of PSD is complex and associated with multiple factors, such as lesion location, monoamine neurotransmitter abnormalities, inflammatory me-chanism, family support, and social handicap. Despite its high prevalence, PSD remains undetected and untreated. PSD has been associated with increased risk of stroke recurrence, affects rehabilitation, is associated with impaired cognitive and functional outcome, and contributes to an increased mortality rate. Therefore, additional attention should be paid to the early diagnosis and effective management of PSD to improve clinical outcomes[1-4].
The DSM IV is widely used to diagnose PSD in clinical studies[5]; however, after the development of the DSM V, scientists and clinicians would prefer these criteria for the diagnosis of PSD. However, the diagnosis of PSD can become difficult for the following reasons:
(1) Language impairment—expressive or receptive;
(2) Cognitive disorders—anosognosia, lack of insight, or awareness of depressive symptoms;
(3) Overlapping symptoms of depression and other medical conditions—many symptoms of depression such as a lack of energy, decreased appetite, and insomnia are found in non-depressed stroke patients due to the hospital environment, medication use, a few other medical conditions, and decreased sleep, energy, concentration, and appetite;
(4) The time duration of onset of major PSD is 9-12 months, while those for minor depression are several years. The major symptoms include guilt, persistent depression, anhedonia, suicidal thoughts, and hopelessness. Prevalence varies with an apparent peak at 3-6 months following stroke and then a decline to about 50% of the initial rates at 1 year. Spontaneous remission can occur 1-2 years after stroke. A large number of individuals develop depression within hours to days after stroke following the pattern that is classically called reactive depression[6-8].
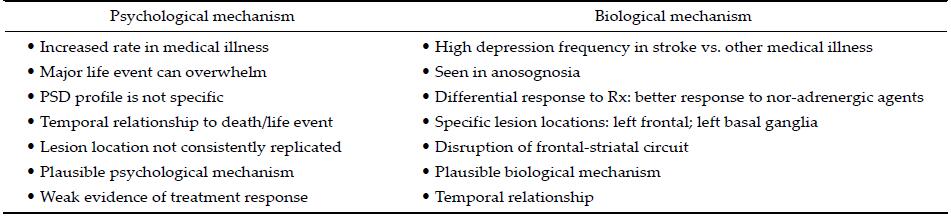
2.2 Etiopathogenesis of PSDThe development of PSD may be due to a proposed biological or psychological mechanism (Table 3). While the biological mechanism includes organic damage to parts of the brain (interruption of neural circuits and neurochemicals), the psychological mechanism includes poor coping skills, for example, a neurotic personality style[2,9-11] (Figure 1). Although each mechanism has its advantages, the etiopathogenesis of PSD is most likely a combination of both biological and psychological mechanisms.

|
| Figure 1 Molecular basis of post-stroke depression[44] |
In one study[12], researchers proved the correlation between the 1-year incidence of PSD and potential risk factors. A complex mixture of pre-stroke social and personal factors as well as stroke-induced emotional, social, and intellectual handicaps was seen. A history of stroke, depression, living alone, pre-stroke social distress, and female sex were significantly related with the development of PSD. A decrease in social activity, intellectual impairment, and pathological crying at 1 month were also associated with PSD.
Stress and depression levels were significantly higher in post-stroke patients who did not exercise regularly. One community-based study[13] confirmed that in-dividuals who exercise regularly had significantly lower stress and depression levels than stroke survivors who did not[14-16].
Apathy is another important NP disorder, and three risk factors including a history of cerebrovascular disease, low HbA1c, and low Mattis dementia rating scale (MDRS) initiation/perseveration (I/P) scores have been associated with this disease.
A low MDRS I/P score is also a risk factor for PSD. These results may be useful to clinicians in recognizing and treating apathy and depression in patients after stroke.
According to the monoamine theory, ischemic lesions disturb the projections ascending from the brainstem and midbrain, resulting in decreased bioavailability of the biogenic amines dopamine, serotonin, and norepinephrine. Involvement of the acetylcholine can also be present[17,18].
2.3 Preventing PSDAntidepressants have been used prophylactically to prevent PSD and physical disorders and reduce mor-tality rates in these patients. However, there is no definitive evidence to prove that psychotherapy or antidepressants can prevent depression. As such, the use of preventive strategies to decrease the risk of mood disorder and thus improve rehabilitation outcomes is critical in the management of post-stroke patients. More studies are required to develop a definite protocol for preventing PSD[19].
2.4 Managing PSDThe early and effective treatment of PSD positively affects the depressive symptoms and rehabilitation outcomes of post-stroke patients. Selective serotonin reuptake inhibitors (SSRIs), antidepressant medications that are commonly prescribed in PSD, could improve stroke outcomes. Treatment with fluoxetine or nortriptyline (NTP) in the first 6 months post-stroke is associated with increased survival rates in depressed and non-depressed individuals. These antidepressants and neurotropics stabilize the chemical imbalance, increase compliance, and may have a positive effect on serotonin-mediated platelet activation. Levels of platelet serotonin transporter are low shortly after stroke.
In another study[20], early treatment (4th week post- stroke) with either NTP or fluoxetine significantly improved the depressed patients' mood, functional and cognitive ability, and neurological function. A close relationship was observed between an appropriate early treatment (including fluoxetine treatment) of PSD and improved neuropsychological rehabilitation. Studies have suggested that, in addition to the benefits of treating PSD, different classes of antidepressants may facilitate the neural mechanisms of recovery in post-stroke individuals. The research to date cannot recommend any protocol of administration of anti-depressant medications for PSD, but it does support the administration of these pharmaceutical interventions on an individual basis.
Exercise also has positive effects on symptoms of depression at the subacute (6 months post-stroke) as well as at chronic stage of recovery (>6 months). There was a significant effect on symptoms when higher-intensity studies were carried out, but not for lower-intensity exercise protocols. Antidepressant use was not observed in the majority of these studies; thus, its interaction with exercise could not be assessed[8,20,21].
In China, one study[22] examined the effect of the early administration of blood-activating and liver-soothing concoctions. Acupuncture was also performed for the patients in that study. This combination of inter-ventions improved depressive symptoms, neurological function, and quality of life in patients with PSD compared to fluoxetine hydrochloride administration. Patients with fewer depressive symptoms have been shown to have higher levels of participation in post- stroke therapy. Results regarding the benefits of early intervention are currently unavailable.
An electro-acupuncture therapy study[23] examined whether it reduces PSD and if motor function impairments interact with the effects of therapy. The results showed that it was effective and reduced PSD (as assessed by the Beck depression inventory and Hamilton depression rating scale), particularly in the good motor function group. These results demonstrate that electro-acupuncture therapy can improve PSD and that the treatment effect varies depending on motor function impairment degree.
PSD has also been treated with serotonin transport inhibitors, suggesting a role for the serotonin system in these patients. One study[24] aimed to determine the levels and their correlation with PSD. The number of platelet serotonin transporters was low shortly after stroke compared with normal subjects, but no difference was found between stroke patients who developed PSD and those who did not. Therefore, a low number of platelet serotonin transporters may be a non-specific state marker for acute stroke and is not directly related with PSD[24,25].
Carotid angioplasty has also shown positive effects in patients with PSD and high-grade carotid stenosis. To assess the differences between carotid angioplasty stent (CAS) placement and antidepressants on PSD, a study[26] of 182 cerebral ischemic stroke patients with high-grade carotid artery stenosis were recruited who were admitted to the Southwest Hospital of the Third Military Medical University, Chongqing, China, between April 2004 and March 2009. The study showed that CAS placement relieved PSD and improved neurologic rehabilitation in patients with high-grade carotid artery stenosis, and the therapeutic effect was superior to that of SSRIs after 1 month.
A transcranial magnetic stimulation (TMS) recently showed promising results in the treatment of PSD. TMS also improved motor recovery and subsequently prevented these individuals from suffering from PSD since impairment of daily activities is one of the risk factors for its development. However, the selection criteria for the use of TMS remain controversial, and TMS frequency and dose remain under consideration and discussion. TMS improves motor function recovery in stroke patients irrespective of the dose administered, but recovery is dependent upon the proportion of the corticomotor integrity. Further research into the use of TMS for PSD is still required[27,28].
It is necessary for PSD to be identified and managed efficiently because of its high symptom burden and association with many other negative health and social consequences. Care, however, must be taken when using antidepressant medications in stroke patients because of the potential for falls, increased bleeding tendency, seizures, and sedation. There is really a need for further research to better define the role of antidepressants in stroke prevention.
3 Post-stroke anxietyThe prevalence of post-stroke anxiety (PSA) is around 20%, and the majority of PSA patients also have PSD or generalized anxiety disorder, defined as a worrying state for at least 6 months plus three other symptoms among restlessness, irritability, lack of energy, poor concentration, muscle tension, and disturbed sleep. A past history of alcohol dependence is also a risk factor for PSA[29,30].
PSA can be divided in early- and late-onset. Early- onset PSA is of shorter duration (1.5 months vs. 3 months) and more strongly related to a premorbid psychiatric history such as alcohol dependence.
In a meta-analysis, NTP appeared to improve PSA. SSRIs are commonly used in PSA, but at present, data of their effect are limited. Most experts suggest avoiding benzodiazepines because of their adverse effects on motor function and cognition.
One study[31] of 98 stroke patients found that:
(1) Anxious depressed group patients had higher rates of cortical lesions than those in the depressed or control group;
(2) The depressed group individuals had a higher frequency of subcortical lesions than the anxious depressed group.
4 Post-stroke catastrophic reactionsCatastrophic reactions (CRs) are outburst of emotion, such as agitation, anxiety, or crying, that occurs when an individual is unable to perform simple tasks that were possible before the stroke event. CRs are common in post-stroke patients.
CRs are often associated with expressive aphasia. PSD and basal ganglia lesions are present in most patients with CR, which may be due to a release phenomenon that results in subcortical damage. Treatment consists of supportive and prophylactic measures. Further studies are required to prepare a protocol for the treatment of post-stroke individuals with CR.
5 Pathological affectPathological affect, also known as emotional incon-tinence or post-stroke emotionalism, is observed in around 15% of post stroke patients. Pathological affect is characterized by frequent or easily provoked out-bursts of laughter or tearfulness that are inappropriate or disproportionate to the situation. Treatment includes antidepressant medications and mood stabilizers to manage pathological crying and laughter. However, more research and studies are required in this area.
6 Post-stroke psychosis/maniaPost-stroke psychosis is a rare complication, and almost all patients who develop it have right front-oparietal lesions and subcortical atrophy compared with controls. Post-stroke psychosis patients also have an increased incidence of post-stroke seizures com-pared to non-psychosis controls. Preexisting psychiatricdisease increased the risk of developing delusional disorders in these patients[32].
Mania following brain pathology is relatively rare. Post-stroke mania has been strongly associated with right hemispheric stroke involving limbic or limbic- related cortical orbitofrontal/basotemporal or subcortical caudate nucleus or thalamus regions[33]. A case of secondary mania late in life with lesions in the dominant hemisphere was also reported in one study[34].
7 Cognitive impairment after strokeIn post-stroke patients, impaired cognition was mostly associated with major cortical syndromes and infarctions in the left posterior and anterior territories of the cerebral artery. According to one study[35], functional impairment was more frequent with cog-nitive impairment, and dependent living post-discharge at a nursing home or in the home was more likely in these post-stroke patients (55.0% with cognitive impairment vs. 32.7% without cognitive impairment, P = 0.001). It was concluded from this study that cognitive impairment occurs frequently in post- stroke individuals and commonly affects orientation, language, memory, and attention.
The presence of cognitive impairment in post-stroke patients has many serious functional consequences in addition to post-stroke physical impairment. Further research is required to address both cognitive and physical impairments[36-39].
8 Recovery from aphasia and neglect after subcortical strokeIn one study[40], cortical regional cerebral perfusion was assessed using N, N, N1-trimethyl-N1-(2)-hydroxy- 3-methyl-5-(I-123) iodobenzyl-1, 3-propanediamine 2 HCl I-123 (HIPDM), and single photon emission computerized tomography in six aphasic and two neglected patients with unilateral subcortical vascular lesions. Assessments were performed both in the acute phase and at 1-6 months post-stroke. In all patients, an almost complete spontaneous recovery was observed that was associated with improved cortical perfusion. In patients with aphasia post-stroke, a correlation was found between aphasia severity and cortical hypoperfusion degree in the acute and follow-up assessments.
9 Neurobiochemical markers of brain damageKinetics and serum concentrations of neuron-specific enolase (NSE) and S100 calcium-binding protein B (S-100B) protein have a high predictive value for early neurobehavioral changes following acute stroke. S-100B protein concentrations at day 2-4 after acute stroke may provide important information about both functional impairment and neurological status at discharge from the acute care hospital[41,42].
NSE and S-100B protein concentrations were sig-nificantly associated with both NIH Stroke Scale scores and infarcted brain area volume. Patients with an adverse neurological outcome had a markedly higher and longer release of both NSE and S-100B protein. Impaired neuropsychological status was related with higher protein S-100B release, but the difference was not statistically significant.
10 Pathological crying after brain injuryFor pathological crying after brain injury, SSRIs are the treatment of choice irrespective of the presence or absence of depression. The tolerability and efficacy of citalopram and paroxetine were compared in a study[43] in which 26 consecutive patients with acquired brain injury had episodes of involuntary crying.
The severity of pathological laughing or crying was rated according to clinical interviews with symptom provocation in those patients. The first 13 patients were treated with paroxetine. Citalopram was administered to the other 13 patients in single daily doses of 10-40 mg. Highly significant (p and lt; 0:001) and rapid-onset (within 1-3 days) improvements of emotionalism were observed after citalopram and paroxetine in those 26 patients. There were no differences in efficacy between citalopram and paroxetine despite the longer duration of symptoms in the citalopram group. Nausea was the only adverse effect observed after paroxetine administration. However, the effect of nausea was reversible in two patients and nausea with vomiting was reversible in another two patients who were on citalopram later. Citalopram was tolerated by all of the patients without any adverse effects[21,43].
11 ConclusionsThe prevalence of NP disorders is relatively high; of them, PSD and PSA are the most common. These syndromes have significant effects on the rehabilita-tion and prognosis of post-stroke patients. Despite these facts, very few studies have examined the treatment of post-stroke neuropsychiatric disorders. NP disorders are not diagnosed in many patients, and late diagnoses are not uncommon.
Clinicians should pay more attention to NP disorders in post-stroke patients. The prevention and efficient management of post-stroke neuropsychiatric disorders have significant positive effects on the outcomes of such individuals. The treatment of NP disorders has the potential to improve quality of life, minimize social handicaps, increase survival rates, and decrease mortality rates during the rehabilitation process.
Since very few studies to date have attempted to develop protocols or guidelines for the treatment or prevention of NS disorders, more studies and further research are required.
Conflicts of interest
All contributing authors declare no conflict of interest.
| [1] | de Man-van Ginkel JM, Hafsteinsdóttir TB, Lindeman E, Ettema RGA, Grobbee DE, Schuurmans MJ. In-hospital risk prediction for post-stroke depression: Development and validation of the post-stroke depression prediction scale. Stroke , 2013, 44 (9) : 2441–2445. DOI:10.1161/STROKEAHA.111.000304 |
| [2] | Hosking SG, Marsh NV. Predictors of depression at one year post-stroke in older adults. Brain Impair , 2013, 14 (3) : 381–391. DOI:10.1017/BrImp.2013.30 |
| [3] | Rajashekaran P, Pai K, Thunga R, Unnikrishnan B. Post-stroke depression and lesion location: A hospital based cross-sectional study. Indian J Psychiatry , 2013, 55 (4) : 343–348. DOI:10.4103/0019-5545.120546 |
| [4] | Zhang WN, Pan YH, Wang XY, Zhao Y. A prospective study of the incidence and correlated factors of post-stroke depression in China. PLoS One , 2013, 8 (11) : e78981. DOI:10.1371/journal.pone.0078981 |
| [5] | Starkstein SE, Lischinsky A. The phenomenology of depression after brain injury. NeuroRehabilitation ,2002, 17(2): 105-113. |
| [6] | Gordon WA, Hibbard MR, Egelko S, Riley E, Simon D, Diller L, Ross ED, Lieberman A. Issues in the diagnosis of post-stroke depression. Rehabil Psychol , 1991, 36 (2) : 71–87. DOI:10.1037/h0079078 |
| [7] | Harrington C, Salloway S. The diagnosis and treatment of post-stroke depression. Med Health R I , 1997, 80 (6) : 181–187. |
| [8] | Robinson RG, Lipsey JR, Price TR. Diagnosis and clinical management of post-stroke depression. Psychosomatics , 1985, 26 (10) : 769–778. DOI:10.1016/S0033-3182(85)72790-9 |
| [9] | Gill RK, Cohen DL. 12 month reviews post stroke-Is there a need. Int J Stroke , 2013, 8 : 33. DOI:10.1111/j.1747-4949.2012.00970.x |
| [10] | Hall P, Hickey A, Mellon L, Brewer L, Horgan F, Dolan E, Shelley E, McGee H, Kelly P, Williams D. ASPIRE-S: Action on secondary prevention interventions and rehabilitation in stroke-A survey of the adequacy of rehabilitation intervention six months post-stroke. Int J Stroke , 2013, 8 : 19. DOI:10.1111/ijs.12010 |
| [11] | Burvill PW, Johnson GA, Chakera TMH, Stewart-Wynne EG, Anderson CS, Jamrozik KD. The place of site of lesion in the aetiology of post-stroke depression. Cerebrovasc Dis , 1996, 6 (4) : 208–215. DOI:10.1159/000108023 |
| [12] | Yang SR, Hua P, Shang XY, Hu R, Mo XE, Pan XP. Predictors of early post ischemic stroke apathy and depression: A cross-sectional study. BMC Psychiatry , 2013, 13 : 164. DOI:10.1186/1471-244X-13-164 |
| [13] | Moore S, Jakovljevic D, Ford GA, Rochester L, Trenell M. The effect of a community exercise intervention on physiological and physical function following stroke: A randomized, controlled trial. Int J Stroke , 2012, 7 : 76. |
| [14] | McDonnell MN, Mackintosh SF, Hillier SL, Bryan J. Regular group exercise is associated with improved mood but not quality of life following stroke. PeerJ , 2014, 2 : e331. DOI:10.7717/peerj.331 |
| [15] | Boss HM, Van Schaik SM, Deijle IA, de Melker EC, van den Berg BT, Scherder EJ, Bosboom WM, Weinstein HC, Van den Berg-Vos RM. Safety and feasibility of post-stroke care and exercise after minor ischemic stroke or transient ischemic attack: MotiveS & MoveIT. NeuroRehabilitation , 2014, 34 (3) : 401–407. |
| [16] | Marzolini S, Oh P, McIlroy W, Brooks D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabil Neural Repair , 2013, 27 (5) : 392–402. DOI:10.1177/1545968312465192 |
| [17] | Chollet F, Acket B, Raposo N, Albucher JF, Loubinoux I, Pariente J. Use of antidepressant medications to improve outcomes after stroke. Curr Neurol Neurosci Rep , 2013, 13 : 318. DOI:10.1007/s11910-012-0318-z |
| [18] | Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L, Popa-Wagner A. Post-stroke depression: Mechanisms, translation and therapy. J Cell Mol Med , 2012, 16 (9) : 1961–1969. DOI:10.1111/jcmm.2012.16.issue-9 |
| [19] | Ramasubbu R. Therapy for prevention of post-stroke depression. Expert Opin Pharmacother , 2011, 12 (4) : 2177–2187. |
| [20] | Salter KL, McClure JA, Kruger H, Foley NC, Teasell RW. Examining adherence to Canadian best practice recommendations for stroke care: Attitudes, beliefs and barriers to the identification and management of post-stroke depression in current practice. Stroke , 2011, 42 : E628. |
| [21] | Wolny T, Saulicz E, Myśliwiec A, Kuszewski M, Kokosz M, Saulicz M, Linek P. Effectiveness of neuromobilisation in upper limb discriminatory sense rehabilitation in late-stage post-stroke patients. Phys Med Rehab Kuror , 2014, 24 (1) : 42–47. DOI:10.1055/s-00000056 |
| [22] | Hu JF, Chen CJ, Bi XL, Yu ZH, Yang PQ, Fan ZJ, Liu Y, Liu TF. Effect of early intervention of liver-smoothing and blood-activating decoction combined with acupuncture on patients with post-stroke depression. China J Chin Mater Med , 2013, 38 (14) : 2403–2405. |
| [23] | He J, Shen PF. Clinical study on the therapeutic effect of acupuncture in the treatment of post-stroke depression. Acupunct Res , 2007, 32 (1) : 58–61. |
| [24] | Rasmussen A, Christensen J, Clemmensen PM, Dalsgaard NJ, Dam H, Hindberg I, Lunde M, Plenge P, Mellerup E. Platelet serotonin transporter in stroke patients. Acta Neurol Scand , 2003, 107 (2) : 150–153. DOI:10.1034/j.1600-0404.2003.02053.x |
| [25] | Golimbet VE, Brusov OS, Factor MI, Zlobina GP, Lezheǐko TV, Lavrushina OM, Petrova EA, Savina MA, Skvortsova VI. Interaction effect of serotonin transporter gene and brain-derived neurotrophic factor on the platelet serotonin content in stroke patients. Zh Nevrol Psikhiatr Im S S Korsakova , 2009, 110 (4 Suppl 2) : 42–45. |
| [26] | Huang H, Chen K, Guo T, Zhang Y, Qu W, Zhou Z, Liu G, Chen L. Treatment with carotid angioplasty stent placement for post-stroke depression compared to antidepressants. Neurosciences (Riyadh) , 2012, 17 (1) : 53–56. |
| [27] | Kwakkel G, Winters C, van Wegen EEH, Nijland RHM, van Kuijk AAA, Visser-Meily A, de Groot J, de Vlugt E, Arendzen JH, Geurts ACH, et al. Effects of unilateral upper limb training in two distinct prognostic groups early after stroke: The EXPLICIT-stroke randomized clinical trial. Neurorehabil Neural Repair , 2016, 30 (9) : 804–816. DOI:10.1177/1545968315624784 |
| [28] | Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol , 2015, 78 (6) : 848–859. DOI:10.1002/ana.24472 |
| [29] | Rao V, Bergey A, Rosenberg P. Sertraline for treatment of post-stroke anxiety. J Neuropsychiatry Clin Neurosci , 2012, 24 (2) : E22. DOI:10.1176/appi.neuropsych.11060134 |
| [30] | Petrova EA, Savina MA, Kontsevoǐ VA, Skvortsova VI. Clinical characteristics of post-stroke anxiety disorders. Zh Nevrol Psikhiatr Im S S Korsakova , 2012, 112 (9) : 12–16. |
| [31] | Starkstein SE, Cohen BS, Fedoroff P, Parikh RM, Price TR, Robinson RG. Relationship between anxiety disorders and depressive disorders in patients with cerebrovascular injury. Arch Gen Psychiatry , 1990, 47 (3) : 246–251. DOI:10.1001/archpsyc.1990.01810150046008 |
| [32] | Devine MJ, Bentley P, Jones B, Hotton G, Greenwood RJ, Jenkins IH, Joyce EM, Malhotra PA. The role of the right inferior frontal gyrus in the pathogenesis of post-stroke psychosis. J Neurol , 2014, 261 (3) : 600–603. DOI:10.1007/s00415-014-7242-x |
| [33] | Prior A, Laursen TM, Larsen KK, Johnsen SP, Christensen J, Andersen G, Vestergaard M. Post-stroke mortality, stroke severity, and preadmission antipsychotic medicine use-A population-based cohort study. PLoS One , 2014, 9 : e84103. DOI:10.1371/journal.pone.0084103 |
| [34] | Celik Y, Erdogan E, Tuglu C, Utku U. Post-stroke mania in late life due to right temporoparietal infarction. Psychiatry Clin Neurosci , 2004, 58 (4) : 446–447. DOI:10.1111/pcn.2004.58.issue-4 |
| [35] | Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: Frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry , 1994, 57 (2) : 202–207. DOI:10.1136/jnnp.57.2.202 |
| [36] | Dong YH, Slavin MJ, Chan BPL, Venketasubramanian N, Sharma VK, Collinson SL, Sachdev PS, Chen CLH. Improving screening for vascular cognitive impairment at three to six months after mild ischemic stroke and transient ischemic attack. Int Psychogeriatr , 2014, 26 (5) : 787–793. DOI:10.1017/S1041610213002457 |
| [37] | Makin SDJ, Turpin S, Dennis MS, Wardlaw JM. Cognitive impairment after lacunar stroke: Systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry , 2013, 84 : 893–900. DOI:10.1136/jnnp-2012-303645 |
| [38] | Schaapsmeerders. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke , 2013, 44 : E81. DOI:10.1161/STR.0b013e31829f6106 |
| [39] | Tu QY, Ding BR, Yang X, Bai S, Tu JS, Liu X, Wang RR, Tao JH, Jin H, Wang YQ, et al. The current situation on vascular cognitive impairment after ischemic stroke in Changsha. Arch Gerontol Geriatr , 2014, 58 (2) : 236–247. DOI:10.1016/j.archger.2013.09.006 |
| [40] | Wunderlich MT, Wallesch CW, Goertler M. Release of neurobiochemical markers of brain damage is related to the neurovascular status on admission and the site of arterial occlusion in acute ischemic stroke. J Neurol Sci , 2004, 227 (1) : 49–53. DOI:10.1016/j.jns.2004.08.005 |
| [41] | Brouns R, De Vil B, Cras P, De Surgeloose D, Mariën P, De Deyn PP. Neurobiochemical markers of brain damage in cerebrospinal fluid of acute ischemic stroke patients. Clin Chem , 2010, 56 (3) : 451–458. DOI:10.1373/clinchem.2009.134122 |
| [42] | Muller U, Murai T, Bauer-Wittmund T, Von Cramon DY. Paroxetine versus citalopram treatment of pathological crying after brain injury. Brain Inj , 1999, 13 (10) : 805–811. DOI:10.1080/026990599121197 |
| [43] | Spalletta G, Bossù P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: A review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry , 2006, 11 (11) : 984–991. DOI:10.1038/sj.mp.4001879 |
| [44] | Schöttke H, Giabbiconi CM. Post-stroke depression and post-stroke anxiety: Prevalence and predictors. Int Psychogeriatr , 2015, 27 (11) : 1805–1812. DOI:10.1017/S1041610215000988 |
| [45] | Mnif L, Sellami R, Masmoudi J. Post-stroke emotional incontinence or bipolar disorder. Neuropsychiatr Dis Treat , 2016, 12 : 1883–1885. DOI:10.2147/NDT |
| [46] | Douven E, Staals J, Schievink S, Kohler S, van Oostenbrugge RJ, Verhey F, Aalten P. Post-stroke fatigue in patients with post-stroke depression and apathy. Int Psychogeriatr , 2015, 27 : S159–S159. |
| [47] | Guiraud V, Gallarda T, Calvet D, Turc G, Oppenheim C, Rouillon F, Mas JL. Depression predictors within six months of ischemic stroke: The DEPRESS study. Int J Stroke , 2016, 11 (5) : 519–525. DOI:10.1177/1747493016632257 |
| [48] | Douven E, Schievink SHJ, Verhey FRJ, van Oostenbrugge RJ, Aalten P, Staals J, Köhler S. The cognition and affect after stroke-A prospective evaluation of risks (CASPER) study: Rationale and design. BMC Neurol , 2016, 16 : 65. DOI:10.1186/s12883-016-0588-1 |
| [49] | Metoki N, Sugawara N, Hagii J, Saito S, Shiroto H, Tomita T, Yasujima M, Okumura K, Yasui-Furukori N. Relationship between the lesion location of acute ischemic stroke and early depressive symptoms in Japanese patients. Ann Gen Psychiatry , 2016, 15 : 12. DOI:10.1186/s12991-016-0099-x |
| [50] | Fang J, Cheng Q. Etiological mechanisms of post-stroke depression: A review. Neurol Res , 2009, 31 (9) : 904–909. DOI:10.1179/174313209X385752 |