文章信息
- 高玉杰, 周妮娜, 李囡, 范洋, 杨志
- Gao Yujie, Zhou Nina, Li Nan, Fan Yang, Yang Zhi
- 11C-MET PET/CT诊断脑胶质瘤术后复发残留的初步研究
- A preliminary study of 11C-methionine PET/CT in diagnosis of recurrence or residue after glioma surgery
- 实用肿瘤杂志, 2021, 36(2): 154-159
- Journal of Practical Oncology, 2021, 36(2): 154-159
-
通信作者
- 周妮娜, E-mail: 13681011804@163.com
-
文章历史
- 收稿日期:2020-02-02
2. 北京大学肿瘤医院核医学科, 北京 100142
2. Department of Nuclear Medicine, Peking University Cancer Hospital, Beijing 100142, China
胶质瘤是最常见的原发性脑恶性肿瘤,已达到中枢神经系统恶性肿瘤总例数的80%以上,成年人发病率为5/10 000。中位生存期仅为8~11个月,5年生存率 < 4%,因其浸润生长的特性,在精确显示肿瘤范围和治疗方面均较困难。高复发率是造成患者死亡率常年居高不下的重要原因之一[1]。
目前一些常规影像学方法(如CT和MRI等)能够提供很好的解剖学信息, 但并不具有特异性,在脑胶质瘤的术前分级、预后判断及脑胶质瘤复发与坏死鉴别等方面也存在一定不足[2]。PET/CT是一种功能影像成像技术,将脑胶质瘤的认识深入到分子影像学水平。目前应用最多的PET显像剂是18F-氟脱氧葡萄糖(18F-fluorodeoxyglucose,18F-FDG)。18F-FDG有助于神经胶质瘤的检测、分级和预后判断[3],但是存在脑本底摄取高,特异度差,对低级别胶质瘤的检出率低,难以准确描绘肿瘤边界等诸多缺点[4]。11C-蛋氨酸(11C-methionine,11C-MET)的出现弥补了18F-FDG PET的一些不足。本文将探讨11C-MET PET/CT在诊断胶质瘤术后复发残留的价值。
1 资料与方法 1.1 一般资料回顾性分析2010年12月至2015年2月因胶质瘤术后行CT或MR检查可疑术后复发残留于北京大学肿瘤医院行11C-MET PET/CT检查的患者17例,所有患者均经二次手术病理或临床随访证实,胶质瘤术后复发残留(postoperative relapse and residue, PORR)14例,治疗后相关良性病变(therapy-related benign changes, TRBC)3例。
1.2 胶质瘤术后复发残留的诊断标准(1)穿刺活检或二次手术病理学诊断证实复发残留。(2)影像学随访 > 2年显示原可疑病灶逐渐扩大,占位效应及周围水肿逐渐加重。符合以上2条中任1条即判定为脑胶质瘤术后复发或残留,反之为TRBC。
1.3 显像方法11C-MET PET/CT检查应用Philips Gemini TF 16行头部采集,注射药物11C-MET 0.15 mci/kg,注射药物后20~30 min显像,PET扫描使用3D模式,时间为10 min。图像衰减矫正使用同机CT图像数据。15 min后行CT扫描。CT扫描条件为视野250 mm、电压140 kV、电流250 mA和螺距0.75,单圈旋转时间0.8 s。将PET以及CT图像传输到工作站进行图像融合成像。
1.4 图像分析图像重建采用滤波反投影法。11C-MET高摄取灶分别由2位有 > 5年PET/CT诊断经验的核医学科医师目测确认,并手动勾画感兴趣区(regions of interest,ROI),由计算机自动生成肿瘤的最大标准摄取值(maximum standardized uptake value,SUVmax),同时在对侧额叶区域勾画2 cm直径大小的ROI,得到正常脑组织的本底平均标准摄取值(mean standard uptake value,SUVmean),并计算靶/本底比值(target/normal background ratio,T/N: 病灶SUVmax/对侧正常额叶SUVmean),并作出定性诊断。比较2位医师病灶半定量测量的一致性,医师之间定性诊断的一致性。PORR组与TRBC组对应的SUVmax(2位医师测量的平均值)作ROC曲线,拟合2位医师定性诊断敏感度及特异度对应的受试者工作特征(receiver operating characteristic,ROC)曲线截断点及SUVmax和曲线下面积。比较PORR组与TRBC组SUVmax及T/N值的差异。胶质瘤PORR组病灶SUVmax及T/N值与胶质瘤世界卫生组织(World Health Organization,WHO)初次手术病理分级行相关性分析。
1.5 统计学分析采用SPSS 20.0软件进行统计学分析。符合正态分布的数据用均数±标准差(x±s)表示。2位医师之间测量值的差异采用配对t检验。PORR组与TRBC组SUVmax及T/N值比较采用独立样本t检验。SUVmax及T/N值与胶质瘤WHO分级关系采用Spearman相关分析。
2 结果 2.1 一般临床情况17例胶质瘤术后患者中,男性10例,女性7例;年龄8~49岁,(35.0±12.1)岁。初次手术病理WHOⅠ级2例(星形1例,发育不良性神经节细胞瘤1例),Ⅱ级7例(星形5例,少突2例),Ⅲ级5例(星形4例,少突1例),Ⅳ级3例(胶质母细胞瘤3例)。病理或随访证实复发残留14例,TRBC 3例(表 1)。
| 临床特征 | PORR组(n=14) | TRBC组(n=3) |
| 性别 | ||
| 男性 | 7 | 3 |
| 女性 | 7 | 0 |
| 年龄(岁,x±s) | 36.2±12.9 | 28.3±2.1 |
| WHO分级 | ||
| Ⅰ级 | 1(发育不良性神经节细胞瘤) | 1(星形细胞瘤) |
| Ⅱ级 | 6(4例星形细胞瘤,2例少突细胞瘤) | 1(星形细胞瘤) |
| Ⅲ级 | 5(4例星形细胞瘤,1例少突细胞瘤) | 0 |
| Ⅳ级 | 2(胶质母细胞瘤) | 1(胶质母细胞瘤) |
| SUVmax(x±s) | 3.79±1.04 | 2.49±0.09* |
| T/N值(x±s) | 2.11±0.92 | 1.32±0.36* |
| 注 PORR:胶质瘤术后复发残留(postoperative relapse and residue);TRBC:治疗后相关良性病变(therapy-related benign changes);WHO:世界卫生组织(World Health Organization);SUVmax:最大标准摄取值(maximum standardized uptake value);T/N:靶/本底比值(target/normal background ratio);*与PORR组比较,P < 0.05 | ||
11C-MET PET头部图像清晰,脑本底(灰质区及基底核区)摄取较低,垂体、泪腺、腮腺和鼻咽可见生理性摄取,作为本底测量区的额叶SUVmax为1.1~2.4。
2.3 2位医师定量测量及定性诊断的一致性分析在半定量测量方面,医师1测量残留或复发病灶SUVmax为2.4~5.5,(3.55±1.05);医师2测量病灶SUVmax为2.4~5.5,(3.56±1.08);两者比较,差异无统计学意义(P=0.57)。采用T/N时,医师1测量值1.06~3.94,(1.96±0.89);医师2测量值1.04~3.92,(1.97±0.90);两者比较,差异无统计学意义(P=0.54)。在定性诊断上,2名医师诊断一致率为100%。
2.4 11C-MET PET/CT对胶质瘤术后复发残留的诊断14例胶质瘤术后复发残留患者中,11C-MET PET图像显示病灶边界清楚的有12例(病灶摄取明显高于周围脑实质本底,图 1~2),病灶边界欠清2例(病灶摄取程度与周围本底相仿)。3例TRBC患者的图像均显示病灶摄取同周围本底相仿(图 3)。
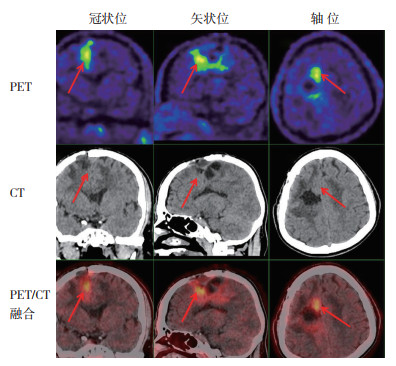
|
| 注 男性,44岁,右顶叶Ⅱ级星形细胞瘤术后4个月复发(随访证实),11C-MET PET/CT显示右顶叶术区周缘放射性浓聚(箭头示),SUVmax为4.1,T/N值为2.80 图 1 Ⅱ级星形细胞瘤术后复发患者的PET/CT检查结果 Fig.1 PET/CT results of patients with postoperative recurrence of gradeⅡ astrocytoma |
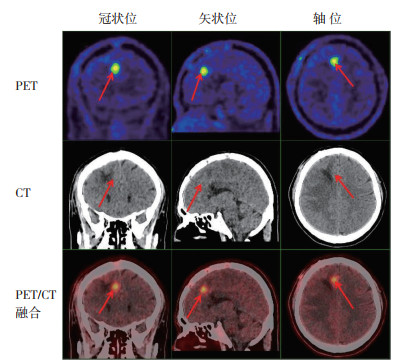
|
| 注 男性,49岁,Ⅲ级星形细胞瘤术后40 d复发(随访证实),术后1周MRI增强未见异常强化区,11C-MET PET/CT显示右额叶代谢活跃灶(箭头示),SUVmax为5.5,T/N值为3.92 图 2 Ⅲ级星形细胞瘤术后复发患者的PET/CT检查结果 Fig.2 PET/CT results of patients with postoperative recurrence of grade Ⅲ astrocytoma |
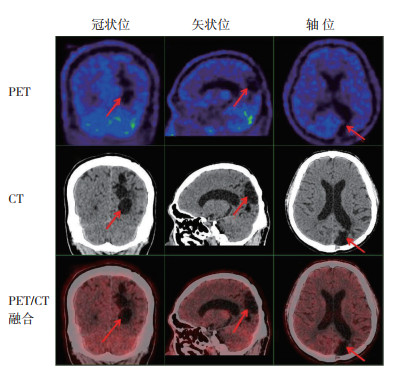
|
| 注 男性,29岁,左侧顶枕部脑胶质瘤术后,部分脑实质缺如(箭头示),术区周围脑实质内见低代谢灶,SUVmax为2.4,T/N值为1.04 图 3 Ⅳ级胶质母细胞瘤术后相关良性病变患者的PET/CT检查结果 Fig.3 PET/CT results of patients with therapy-related benign changes of grade Ⅳ glioblastoma |
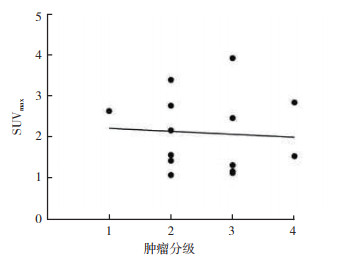
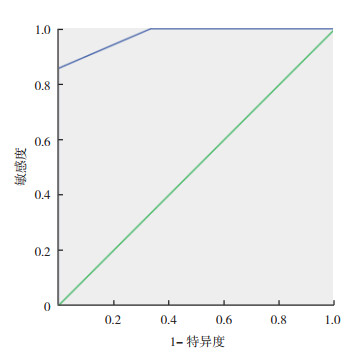
复发或残留胶质瘤病灶的SUVmax与T/N有相关性(r=0.86,P < 0.01);PORR组与TRBC组SUVmax及T/N值比较,差异均具有统计学意义(均P < 0.05,图 4),SUVmax与肿瘤WHO分级无相关性(r=0.102,P=0.728)(图 5);T/N值与肿瘤WHO分级亦无相关性(r=0.056,P=0.848)。11C-MET PET/CT诊断胶质瘤术后复发的敏感度为85.7%(12/14),特异度为100.0%(3/3),准确率为88.2%(15/17)。拟合ROC曲线肿瘤SUVmax界值为2.65,曲线下面积(area under curve,AUC)为0.976(图 6)。
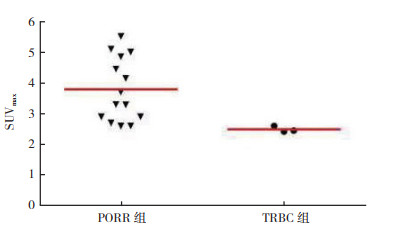
|
| 图 4 PORR组与TRBC组11C-MET PET病灶SUVmax Fig.4 SUVmax values of lesions by 11C-MET PET in the PORR and TRBC groups |
|
| 注 r=0.102,P=0.728 图 5 PORR组肿瘤分级与病灶SUVmax相关性对应散点图 Fig.5 Scatter plot of the correlation between tumor grade and SUVmax in the PORR group |
|
| 注 拟合ROC曲线肿瘤SUVmax界值为2.65,AUC=0.976 图 6 诊断胶质瘤术后复发敏感度与特异度的ROC曲线 Fig.6 ROC curve of sensitivity and specificity for the diagnosis of glioma recurrence after surgery |
胶质瘤发生于神经外胚层,2016版WHO新分类首次提出分子病理对肿瘤分类的影响,打破仅依靠显微镜对脑肿瘤进行分类的原则,将分子信息整合进入脑肿瘤诊断,将弥漫性星形细胞和少突胶质细胞肿瘤作为一大类进行讨论。但在分级上,根据细胞核的改变、核分裂像、内皮细胞增殖及坏死情况仍将胶质瘤分为4级[5-6]。
11C-MET PET最先于1983年被报道作为一种有潜力的成像技术来显示胶质瘤[7]。11C-MET易穿透血-脑脊液屏障(blood-brain barrier,BBB)进入脑组织,健康成人大脑内的神经元多为分化终末细胞,没有明显的蛋白质合成代谢,因此,正常脑组织对11C-MET呈低摄取。脑胶质瘤组织中,细胞恶性增殖,蛋白质和RNA的合成加速,对11C-MET的摄取增加,这也是氨基酸正电子显像的理论基础。之后的研究也证明,这种方法在病灶的检出上优于18F-FDG PET[8-9]。但是其短的半衰期(20 min)限制了其临床广泛应用。
3.1 11C-MET PET显像在脑胶质瘤分级中的作用脑胶质瘤的恶性程度不同其临床表现、治疗方法及预后均不相同,低级别胶质瘤(WHOⅠ~Ⅱ级)治疗以手术为主,尽量切除全部瘤灶,术后辅以放疗,预后较好;高级别胶质瘤(WHO Ⅲ~Ⅳ级)呈浸润生长,有时不能切除全部病灶,而只要求切除部分瘤灶,术后再进行放疗控制残余肿瘤组织,预后不良。因此,术前对脑胶质瘤作出准确的分级诊断对指导治疗和判断预后十分重要。
18F-FDG PET与11C-MET PET对于胶质瘤分级方面的报道存在争议。有些研究报道,18F-FDG在胶质瘤分级上优于11C-MET[10-11]。18F-FDG可以通过比较肿瘤不同区域代谢高低的差别,指导活检部位选择[5]。然而Takano等[12]报道对于不强化的胶质瘤,11C-MET在鉴别WHOⅡ级与Ⅲ级胶质瘤上优于18F-FDG,对于11C-MET在胶质瘤分级上的应用持肯定态度。Nariai等[13]的研究中194例患者应用T/N这一半定量指标,发现在低级别与高级别胶质瘤组之间差异具有统计学意义(P < 0.05),但在Ⅰ级与Ⅱ级患者之间及Ⅲ级与Ⅳ级患者之间比较,差异均无统计学意义(均P > 0.05)。Torii等[14]分析67例胶质瘤11C-MET显像结果,11C-MET摄取与Ki-67无明显相关性(r=-0.35,P=0.832)。对其中28例星形细胞单独进行分析,发现11C-MET摄取的T/N值与Ki-67相关(r=0.426,P=0.023)。11C-MET摄取可以提示星形细胞瘤的良恶性,但不能提示胶质瘤。Hatakeyama等[15]发现,虽然11C-MET SUVmax和T/N比值在Ⅱ级和Ⅳ级胶质瘤中比较,差异均具有统计学意义(均P < 0.01),但是高低级别的11C-MET摄取有很大重叠,且11C-MET SUVmax和T/N比值在Ⅱ级和Ⅲ级胶质瘤间比较,差异均无统计学意义(均P > 0.05),有少突胶质瘤成分的低级别肿瘤11C-MET摄取比较高。理论上MET的摄取不仅受特异的载体介导的主动转运的影响,而且也受因BBB破坏而产生的被动扩散的影响。本文对11C-MET SUVmax和T/N值分别与WHO初次手术分级行相关分析,均未发现有相关性(r=0.102,P=0.728;r=0.056,P=0.848),分析其原因为手术对BBB的破坏影响11C-MET摄取以及本研究纳入的胶质瘤有3例少突胶质瘤成分并且胶质瘤不同级别之间有很大的重叠,深入研究需要大样本的胶质瘤亚分类研究。
3.2 11C-MET在诊断胶质瘤复发与残留中的作用由于手术或放疗等其他治疗手段导致BBB的损伤,使得增强CT或者MRI在鉴别放疗后损伤或复发上存在较大难度。氨基酸类显像剂在代谢水平进行显像,可以提供更准确的信息[16]。脑胶质瘤复发的11C-MET摄取量高于放射性脑损伤,推测是由于肿瘤细胞处于异常增殖状态导致代谢率大幅提升,病灶局部蛋白质合成旺盛,11C-MET富集于此[17]。对比11C-MET与18F-FDG在检出肿瘤复发中的作用的研究发现,30例原发脑胶质瘤的患者中,28例11C-MET有高摄取,而17例18F-FDG有高摄取[18]。研究发现,采用11C-MET动态模式采集可以鉴别胶质瘤病灶和肉芽肿性病变:肉芽肿对11C-MET摄取在最初分布后呈1个指数级的下降,而胶质瘤病灶则逐渐上升;具体表现形式(2 min的SUVmax与15 min的SUVmax比值)为:肉芽肿病变为 > 1.1,肿瘤病灶为 < 1.03,两者比较,差异具有统计学意义(P < 0.01)[19]。
一项荟萃分析也表明,11C-MET在诊断胶质瘤治疗后复发上有较高的诊断准确性,诊断胶质瘤复发的总的敏感度和特异度分别为0.88和0.85[20]。并且指出最常用评价复发的参数为肿瘤组织平均T/N值(T/Nmean),第2个常用的评价参数为肿瘤组织的最大T/N值(T/Nmax)。而本研究11C-MET在诊断胶质瘤术后复发上的敏感度为85.7%,特异度为100%,准确率为88.2%。拟合ROC曲线对应的SUVmax界值为2.65。11C-MET PET在诊断胶质瘤术后复发上是一个值得推广应用的较好的检查手段。而其在胶质瘤分级的应用上持怀疑态度,需要进一步进行大样本的胶质瘤亚分类研究证实。
综上所述,本研究显示,11C-MET PET/CT脑本底摄取低,能清楚显示病灶,不同医师之间诊断有很高的一致性。诊断准确率较高,易于胶质瘤术后残留或复发的检出;能清楚显示病灶边界,易于病灶活检或靶区勾画。病灶的11C-MET摄取程度与肿瘤分级无相关性,有利于低级别肿瘤复发的检出。
| [1] |
Shaw TB, Jeffree RL, Thomas P, et al. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of glioma[J]. J Med Imaging Radiat Oncol, 2019, 63(5): 650-656. DOI:10.1111/1754-9485.12929 |
| [2] |
折刚刚, 郝文炯. 磁共振波谱在脑胶质瘤边界确定中的研究进展[J]. 实用肿瘤杂志, 2019, 34(1): 7-10. |
| [3] |
Di Chiro G, DeLaPaz RL, Brooks RA, et al. Glucose utilization of cerebral gliomas measured by 18F fluorodeoxyglucose and positron emission tomography[J]. Neurology, 1982, 32(12): 1323-1329. DOI:10.1212/WNL.32.12.1323 |
| [4] |
Takahashi M, Soma T, Mukasa A, et al. Pattern of FDG and MET distribution in high- and low-grade gliomas on PET images[J]. Clin Nucl Med, 2019, 44(4): 265-271. DOI:10.1097/RLU.0000000000002460 |
| [5] |
Moreau A, Febvey O, Mognetti T, et al. Contribution of different positron emission tomography tracers in glioma management: focus on glioblastoma[J]. Front Oncol, 2019, 9: 1134. DOI:10.3389/fonc.2019.01134 |
| [6] |
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary[J]. Acta Neuropathologica, 2016, 131(6): 803-820. DOI:10.1007/s00401-016-1545-1 |
| [7] |
Bergstrom M, Collins VP, Lundqvist H, et al. Discrepancies in brain tumor extent as shown by computed tomography and positron emission tomography using 68Ga EDTA, 11C glucose, and 11C methionine[J]. J Comput Assist Tomogr, 1983, 7(6): 1062-1066. DOI:10.1097/00004728-198312000-00022 |
| [8] |
Chung J, Kim Y, Kim S, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET[J]. Eur J Nucl Med Mol Imaging, 2002, 29(2): 176-182. DOI:10.1007/s00259-001-0690-4 |
| [9] |
Zhao C, Zhang Y, Wang J. A meta-analysis on the diagnostic performance of 18F-FDG and 11C-methionine PET for differentiating brain tumors[J]. Am J Neuroradiol, 2014, 35(6): 1058-1065. DOI:10.3174/ajnr.A3718 |
| [10] |
Singhal T, Narayanan T, Jain V, et al. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas[J]. Mol Imaging Biol, 2008, 10(1): 1-18. DOI:10.1007/s11307-007-0115-2 |
| [11] |
Manabe O, Hattori N, Yamaguchi S, et al. Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging[J]. Eur J Nucl Med Mol Imaging, 2015, 42(6): 896-904. DOI:10.1007/s00259-015-2996-7 |
| [12] |
Takano K, Kinoshita M, Arita H, et al. Diagnostic and prognostic value of 11C-methionine PET for nonenhancing gliomas[J]. Am J Neuroradiol, 2016, 37(1): 44-50. DOI:10.3174/ajnr.A4460 |
| [13] |
Nariai T, Tanaka Y, Wakimoto H, et al. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma[J]. J Neurosurg, 2005, 103(3): 498-507. DOI:10.3171/jns.2005.103.3.0498 |
| [14] |
Torii K, Tsuyuguchi N, Kawabe J, et al. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas[J]. Ann Nucl Med, 2005, 19(8): 677-683. DOI:10.1007/BF02985116 |
| [15] |
Hatakeyama T, Kawai N, Nishiyama Y, et al. 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma[J]. Eur J Nucl Med Mol Imaging, 2008, 35(11): 2009-2017. DOI:10.1007/s00259-008-0847-5 |
| [16] |
García JR, Baquero M, Bassa P, et al. Diagnosis of recurrent glioma and antiangiogenic treatment response by 11C-Methionine PET[J]. Rev Esp Med Nucl Imagen Mol, 2015, 34(6): 398-399. |
| [17] |
Nestor M, Julio J, Isabel M, et al. Value of the visual and semiquantitative analysis of carbon-11-methionine PET/CT in brain tumors' recurrence versus post-therapeutic changes[J]. Nucl Med Commun, 2017, 38(12): 1125-1132. DOI:10.1097/MNM.0000000000000754 |
| [18] |
Van Laere K, Ceyssens S, Van Calenbergh F, et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value[J]. Eur J Nucl Med Mol Imaging, 2005, 32(1): 39-51. DOI:10.1007/s00259-004-1564-3 |
| [19] |
Songji Z, Yuji K, Min Y, et al. Dynamic 11C-methionine PET analysis has an additional value for differentiating malignant tumors from granulomas: an experimental study using small animal PET[J]. Eur J Nucl Med Mol Imaging, 2011, 38(10): 1876-1886. DOI:10.1007/s00259-011-1865-2 |
| [20] |
Xu W, Gao L, Shao A, et al. The performance of 11C-Methionine PET in the differential diagnosis of glioma recurrence[J]. Oncotarget, 2017, 8(53): 91030-91039. DOI:10.18632/oncotarget.19024 |
 2021, Vol. 36
2021, Vol. 36


