文章信息
- 张玉琴, 陈章兴, 戴益琛, 朱小三, 詹晓娟, 傅育卡, 吴婧
- Zhang Yuqin, Chen Zhangxing, Dai Yichen, Zhu Xiaosan, Zhan Xiaojuan, Fu Yuka, Wu Jing
- Pokemon在缺氧微环境中对肝细胞癌血管形成的作用
- Eff ect of Pokemon on angiogenesis of hepatocellular carcinoma under hypoxia microenvironment
- 实用肿瘤杂志, 2022, 37(5): 444-449
- Journal of Practical Oncology, 2022, 37(5): 444-449
基金项目
- 厦门市医疗卫生科技计划项目(3502Z20184067)
-
通信作者
- 陈章兴, E-mail: chenzx2019@126.com
-
文章历史
- 收稿日期:2021-10-29
肝细胞癌是全世界常见的六大恶性肿瘤之一,病死率排第4位[1]。肝细胞癌发生和发展与肿瘤细胞增殖和凋亡密切相关。目前已有研究证实,鼠双微体2(murine double minute 2,MDM2)-p53通路及白细胞介素-6(interleukin-6,IL-6)、转化生长因子α(transforming growth factor α,TGFα)、转录因子NF-κβ和裂解刺激因子亚单位2(cleavage stimulation factor subunit 2,CSTF2)基因均参与肝癌的发生和发展[2-4]。除此之外,肿瘤血管形成在肿瘤发生和发展中扮演重要角色。肝癌是血管增生性肿瘤,其肿瘤血管丰富[5-6]。血管内皮生长因子(vascular endothlial growth factor,VEGF)是正常血管形成及肿瘤微血管形成的关键基因,在多种肿瘤中高表达[7]。而缺氧微环境是实体肿瘤中的常见现象,其与肿瘤细胞的血管形成密切相关。缺氧环境下,缺氧诱导因子1(hypoxia inducible factor-1,HIF-1)表达增加,可调控VEGF表达影响肿瘤血管形成[8]。前期研究发现,Pokemon在肝癌组织中高表达,经丝氨酸/苏氨酸蛋白激酶(protein kinase B,AKT)/细胞外调节蛋白激酶(extracellular regulated protein kinase,ERK)信号通路促进肝癌细胞增殖并经死亡受体介导的凋亡途径(Fas-FADD-caspase8途径)和线粒体途径介导的细胞凋亡通路抑制肝癌细胞的凋亡[9-10]。然而针对Pokemon对肝细胞癌血管形成的作用的相关研究甚少。本研究探讨Pokemon在缺氧条件下对肝细胞癌血管形成的影响。
1 材料与方法 1.1 细胞株及主要试剂和仪器设备人肝细胞癌细胞株HepG2购自美国ATCC。人肝细胞癌细胞株Huh7及人宫颈癌细胞株Hela购自中国科学院上海细胞库。TRIzol和一步法RNA反转录试剂盒购自美国Invitrogen公司。脂质体2000转染试剂、胎牛血清、100×双抗、DMEM和RPMI-1640培养液均购自美国Gibco公司。荧光定量PCR仪(ABI prism7500)和PCR仪(Gene Amp2700)均购自美国Applied Biosystems公司。蛋白电泳仪和蛋白转移装置均购自美国Biorad公司。核酸蛋白检测仪购自德国Eppendorf公司。Pokemon抗体和GAPDH抗体购自英国Abcam公司。HIF1α抗体和VEGFR抗体购自美国CST公司。
1.2 细胞培养HepG2细胞采用含10%胎牛血清的DMEM培养液进行培养。Huh7细胞采用含10%胎牛血清的RPMI-1640培养液。两者均为贴壁生长型的细胞。将细胞接种于无菌培养皿内,并放置于37℃、5%CO2、100%湿度的细胞培养箱中培养。
1.3 构建细胞培养缺氧微环境缺氧产气袋AnaeroPack及配套2.5 L密封容器购自日本三菱公司,其基本原理是将密闭空间中的氧气完全吸收掉,然后产生二氧化碳,开发出完全厌氧培养环境(AneroPack-Anaero,30 min反应后氧气浓度降为0)。将培养皿放入装有厌氧产气袋的密闭容器,构建缺氧的培养环境,再放入细胞培养箱中培养。
1.4 实时荧光定量PCR检测实验所用引物由北京华大基因有限公司合成(引物序列见表 1)。采用SYBR Green Ⅰ试剂进行检测;收获HepG2和Huh7细胞后,提取细胞总RNA,反转录为cDNA;循环参数:95℃ 5 min,94℃ 40 s,60℃ 40 s,72℃ 40 s;共40个循环,最后72℃ 10 min。
| 基因 | 引物序列 |
| Pokemon | F:5'-GGGGACAGCGACGAGGAG-3' |
| R:5'-CGTAGTTGTGGGCAAAGG-3' | |
| HIF-1α | F:5'-TATGAGCCAGAAGAACTTTTAGGC-3' |
| R:5'-CACCTCTTTTGGCAAGCATCCTG-3' | |
| VEGFA | F:5'-TTGCCTTGCTGCTCTACCTCCA-3' |
| R:5'-GATGGCAGTAGCTGCGCTGATA-3' | |
| VEGFR2 | F:5'-GGAACCTCACTATCCGCAGAGT-3' |
| R:5'-CCAAGTTCGTCTTTTCCTGGGC-3' | |
| ANG2 | F:5'-TCCTATGGAGGACATTCCGACG-3' |
| R:5'-CTGCAACACCATCAATGGCGGA-3' | |
| GAPDH | F:5'-CGACAGTCAGCCGCATCTT-3' |
| R:5'-CCCCATGGTGTCTGAGCG-3' | |
| 注 HIF-1α:缺氧诱导因子-1α(hypoxia inducible factor-1α);VEGFA:血管内皮生长因子A(vascular endothelial growth factor A);VEGFR2:血管内皮生长因子受体2(vascular endothelial growth factor receptor 2);ANG2:促血管生成素2(angiopoietin-2);GAPDH:甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase);F: 上游引物;R: 下游引物 | |
RIPA裂解液提取细胞总蛋白,Bradford法检测蛋白浓度,采用恒压电泳及恒流电转将蛋白转移至PVDF膜上,用5%无脂奶粉封闭PVDF膜,孵育一抗(抗体按1∶1 000稀释于BSA液中,加入抗体水平摇床4℃孵育过夜),次日用TBS(含0.1%Tween20)洗涤PVDF膜3次(水平摇床,每次洗膜10 min);孵育二抗(将膜浸泡于按1∶5 000稀释的二抗中,室温下水平摇床缓慢摇动孵育1 h),用TBS洗涤PVDF膜3次(水平摇床,每次洗膜10 min),最后使用ECL法显影。
1.6 靶向Pokemon基因干扰质粒构建及验证运用RNA干扰技术(RNAi),成功构建靶向Pokemon基因的siRNA重组质粒psiRNA。psiRNA上游引物:5'-CACCAGTAGAATGTGTACGGGATACGTGTGCTGTCCGTATCTCGTCACGTTCTGCTTTTTT-3',下游引物:5'-GCATAAAAAGCAGAACGTGTACGAGATACGGACAGCACACGTATCCCGTACACATTCTACT-3'。将干扰质粒psiRNA和PU6空载质粒分别稳定转染HepG2和Huh7细胞,利用嘌呤霉素筛选出稳转细胞株HepG2 si-Pokemon、HepG2-PU6(对照)、Huh7 si-Pokemon和Huh7-PU6(对照),并在蛋白水平验证Pokemon的干扰效果。
1.7 构建过表达HIF-1α的Hela细胞培养HeLa细胞密度约占培养板的80%,进行转染,转染前1 d细胞换新的培养液;用250 μL无血清培养液稀释HIF-1α质粒(0、5、10和15 μg),轻轻混匀;用无血清培养液稀释LipofectamineⓇ 2000(0、12.5、25和37.5 μL)至250 μL,轻轻混匀,室温孵育5 min;将质粒稀释液和转染试剂稀释液混匀,室温孵育20 min;将复合物加入培养板中,放入37℃ CO2培养箱孵育36 h,最后用Western blot验证。
1.8 统计学分析采用Graphpad Prism软件进行数据分析。组间比较采用t检验。以P < 0.05为差异具有统计学意义。
2 结果 2.1 肝细胞癌细胞Pokemon的表达及沉默效果验证HepG2和Huh7细胞中Pokemon均高表达(图 1)。采用RNA沉默技术构建稳定沉默Pokemon基因的稳转细胞株(HepG2 si-Pokemon和Huh7 si-Pokemon)。Western-blot法显示,HepG2 si-Pokemon和Huh7 si-Pokemon细胞株中Pokemon表达受到抑制(均P < 0.05,图 1)。
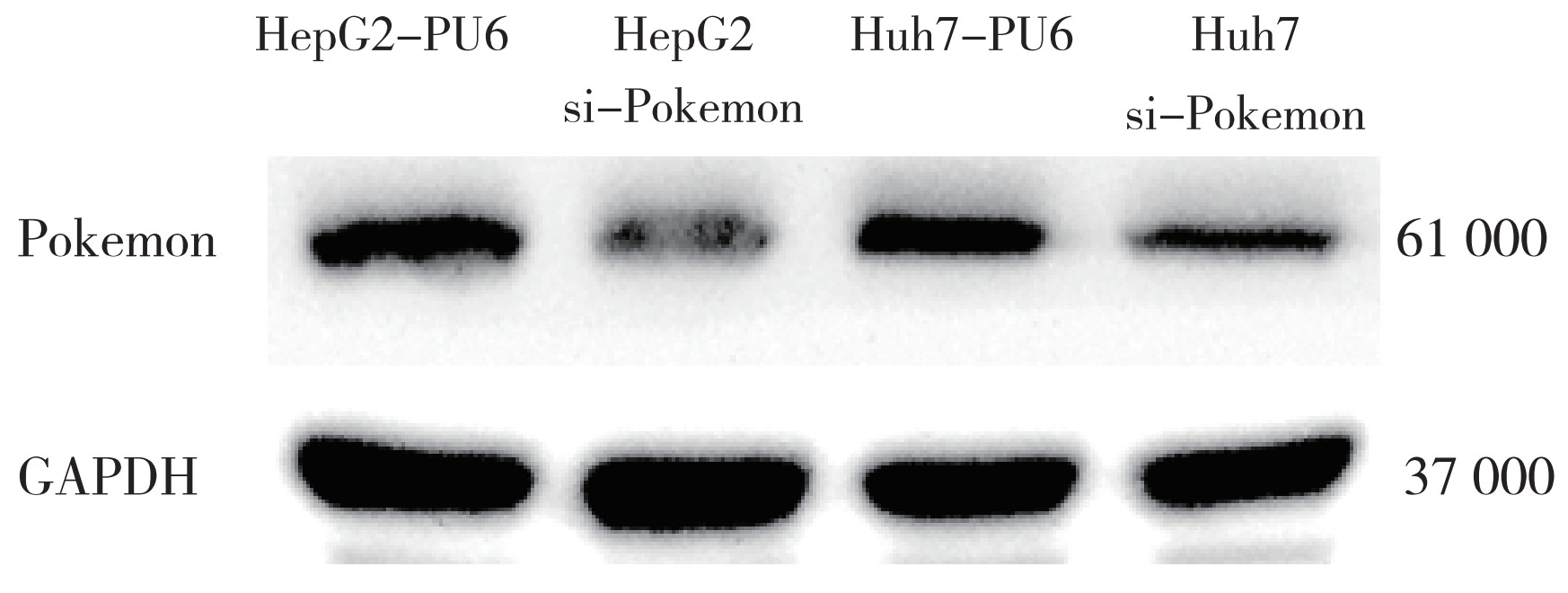
|
| 图 1 Western blot验证稳转肝细胞癌细胞株中Pokemon的干扰效率 Fig.1 Western blot analysis confirmed siRNA-mediated Pokemon silencing in hepatocellular carcinoma cells |
RT-PCR检测显示,与HepG2-PU6细胞比较,HepG2 si-Pokemon细胞中血管形成相关基因(ANG2、VEGFA和VEGFR2)的表达受到抑制,差异均具有统计学意义(均P < 0.05)。与Huh7-PU6细胞比较,Huh7 si-Pokemon细胞中ANG2、VEGFA和VEGFR2基因的表达也受到抑制(均P < 0.05,图 2)。
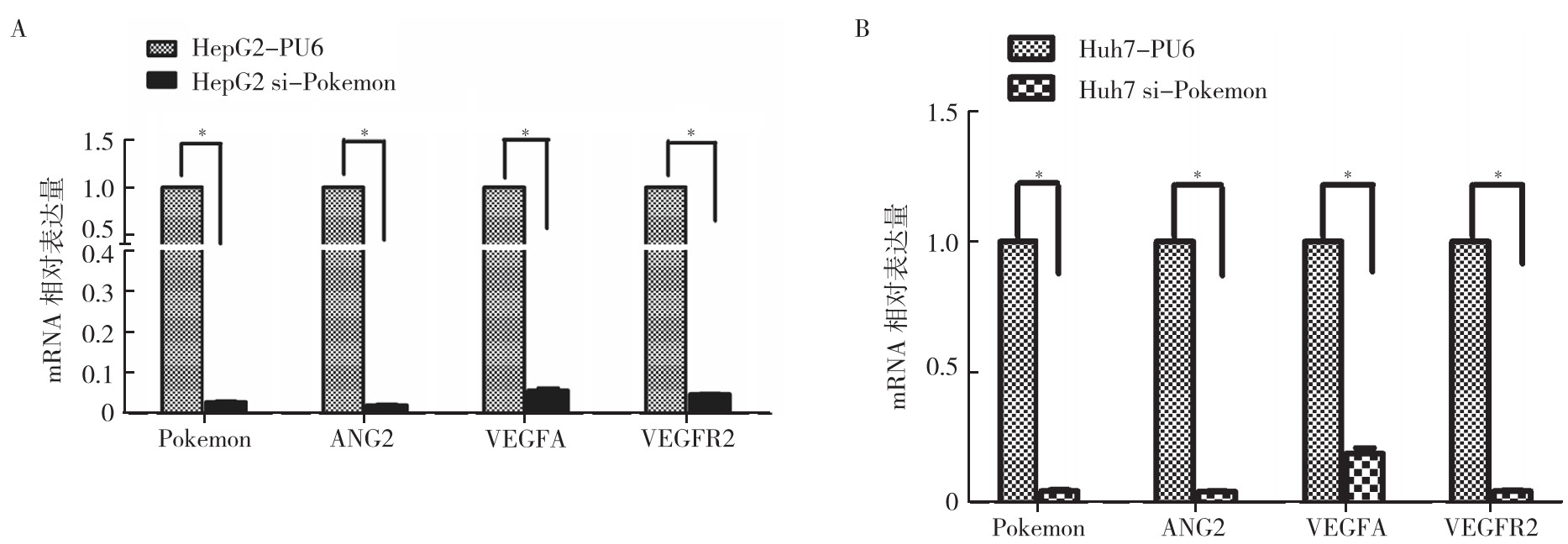
|
| 注 A:HepG2细胞Pokemon沉默后血管形成相关基因表达变化;B:Huh7细胞Pokemon沉默后血管形成相关基因表达变化;ANG2:促血管生成素2(angiopoietin-2);VEGFA:血管内皮生长因子A(vascular endothelial growth factor A);VEGFR2:血管内皮生长因子受体2(vascular endothelial growth factor receptor 2);*P < 0.05 图 2 RT-PCR检测沉默Pokemon的肝细胞癌细胞株HepG2和Huh7中血管形成相关基因表达的变化 Fig.2 Expressions of angiogenesis related genes in hepatocellular carcinoma HepG2 and Huh7 cells with Pokemon silenced detected by RT-PCR |
Western blot法检测显示,缺氧条件下,HepG2 si-Pokemon细胞培养2、4和6 h后,HIF-1α的表达均较HepG2-PU6细胞减少(P < 0.05,图 3)。缺氧条件下,Huh7 si-Pokemon细胞培养6 h后,HIF-1α的表达较Huh7-PU6细胞减少(P < 0.05)。
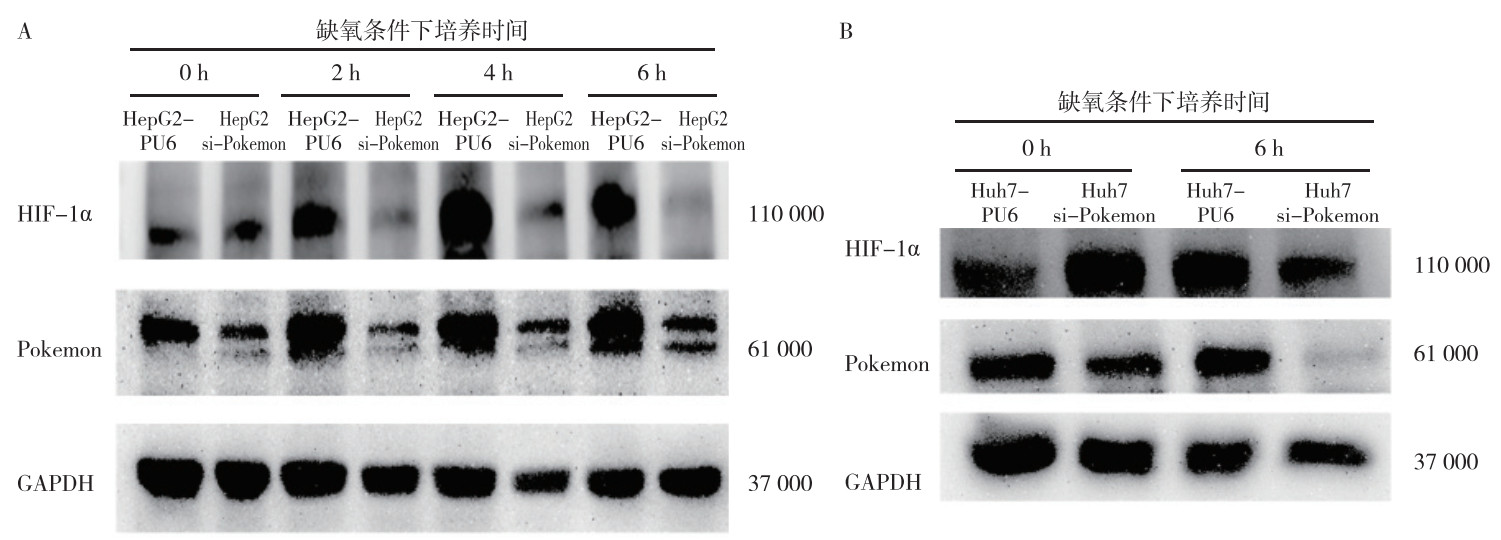
|
| 注 A:HepG2-PU6和HepG2 si-Pokemon细胞中HIF-1α和Pokemon的蛋白表达;B:Huh7-PU6和Huh7 si-Pokemon细胞中HIF-1α和Pokemon的蛋白表达;HIF-1a:缺氧诱导因子-1α(hypoxia inducible factor-1α);GAPDH:甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase) 图 3 Western blot法检测缺氧条件下肝细胞癌细胞HepG2 si-Pokemon、HepG2-PU6、Huh7 si-Pokemon和Huh7-PU6中HIF-1α和Pokemon的蛋白表达 Fig.3 Expression of HIF-1α and Pokemon in hepatocellular carcinoma cells HepG2 si-Pokemon, HepG2-PU6, Huh7 si-Pokemon, and Huh7-PU6 detected by Western blot under hypoxia condition |
而在Hela细胞中采用0、5、10和15 μg HIF-1α质粒瞬转36 h后,Western blot检测发现,随着HIF-1α的表达增加,Pokemon蛋白的表达变化差异无统计学意义(P > 0.05,图 4)。缺氧条件下,沉默Pokemon基因可抑制HIF-1α的表达,而过表达HIF-1α并不能调控Pokemon的表达。
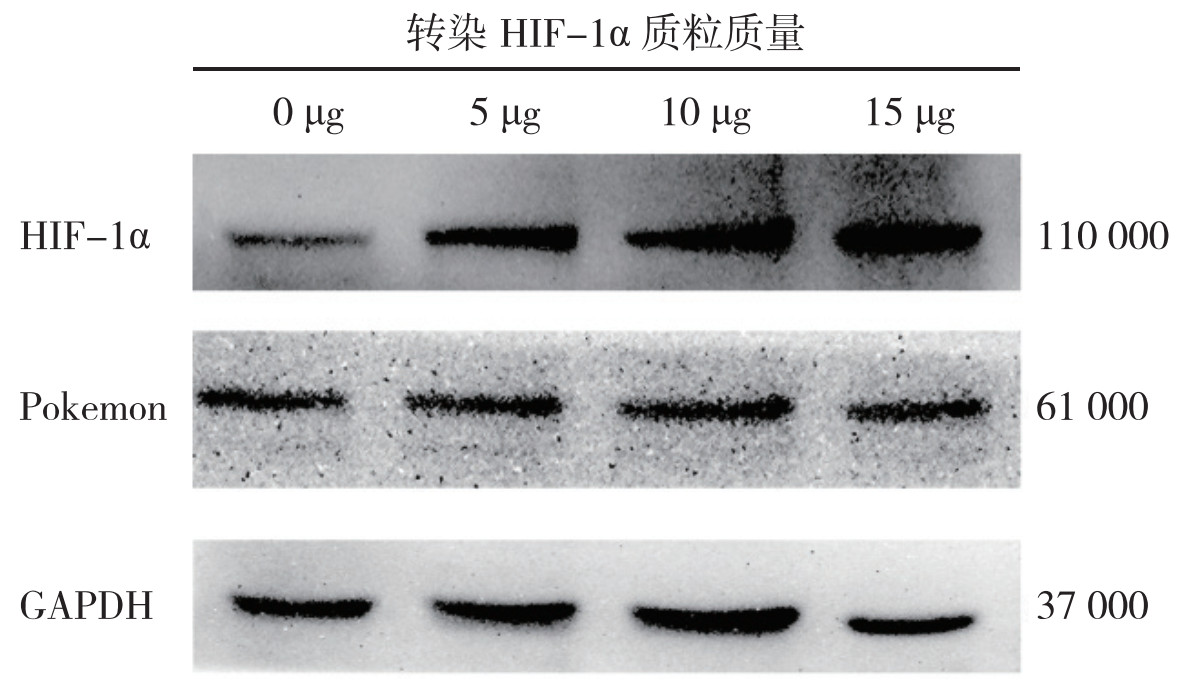
|
| 注 HIF-1a:缺氧诱导因子-1α(hypoxia inducible factor-1α);GAPDH:甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase) 图 4 Western blot检测过表达HIF-1α的HeLa细胞中Pokemon蛋白表达变化 Fig.4 Expression of Pokemon in Hela cells with HIF-1α overexpression detected by Western blot |
Pokemon是具有原癌基因活性的转录抑制因子,是POZ and Kruppel(POK)家族中的成员之一,其包括C末端的Kruppel型结构域和N末端的POZ/BTB结构域。N末端结构域可为蛋白招募联合抑制因子,从而起到转录抑制作用。Pokemon基因与多种肿瘤如结肠癌、乳腺癌、前列腺癌和甲状腺肿瘤的发生和发展密切相关[11-14]。研究发现,Pokemon通过多种分子机制促进肿瘤发生和发展:(1)Pokemon可抑制ARF的转录活性,从而激活MDM2,进一步抑制下游靶基因p53的表达,导致肿瘤发生[15];(2)Pokemon可抑制阻滞细胞周期的关键因子p21,调控细胞周期,促进肿瘤的发生和发展[16];(3)Pokemon促进NF-κB入核及定位,并促进NF-κB应答基因E-selectin的转录活性,从而导致肿瘤的发生[17]。而在肝细胞癌中,目前已有研究证实,原癌基因Pokemon可通过促进肝癌细胞的增殖及抑制其凋亡参与肿瘤发生和发展[9-10]。但是Pokemon对肿瘤细胞血管形成相关方面的机制研究较少,而本实验结果显示,Pokemon在肝细胞癌血管形成中起着关键性作用。
肝癌细胞代谢旺盛,需要依赖肿瘤新生血管来供给营养,因此肿瘤血管形成在肝癌细胞中增殖、侵袭和转移中起着至关重要的作用[18]。促血管生成因子VEGF和VEGFR在肝细胞癌血管形成中起着关键性的作用[6, 19]。肿瘤缺氧微环境是固体肿瘤中常见现象,也常见于肝细胞癌中[20]。其可诱导HIF-1α的形成。HIF-1α作为低氧适应性介导者,可活化血管形成信号通路的相关基因包括VEGF和VEGFR[21]。在肝癌中血管形成相关基因ANG2、VEGFA和VEGFR2是高表达的[22-23],也有临床研究显示,VEGF与肝癌进展及临床预后差高度相关[21, 24]。本研究显示,在抑制Pokemon基因的表达后,血管形成相关基因的表达均受到抑制,说明Pokemon基因与肝癌细胞血管形成正相关。为了研究其调控的相关分子机制,本研究模拟了肝癌细胞生存的缺氧微环境,证实了Pokemon可调控HIF-1α的表达。在肝癌细胞中,Pokemon可能通过上调HIF-1α的表达而促进肝癌细胞的血管形成。
综上所述,Pokemon不仅参与肝癌细胞增殖和凋亡过程。本研究也证实,Pokemon可参与肝癌细胞血管形成。目前肝癌的治疗主要有手术、射频消融、介入靶向和免疫疗法[25-26],结合本研究结果,Pokemon基因有望成为肝细胞癌的生物学标志物及治疗的有效靶点,对肝细胞癌的诊断、疗效预测及预后起关键性作用。
| [1] |
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI:10.3322/caac.21492 |
| [2] |
Cao H, Chen XS, Wang ZJ, et al. The role of MDM2-p53 axis dysfunction in the hepatocellular carcinoma transformation[J]. Cell Death Discov, 2020, 6: 53. |
| [3] |
Alqahtani A, Khan Z, Alloghbi A, et al. Hepatocellular carcinoma: molecular mechanisms and targeted therapies[J]. Medicina (Kaunas), 2019, 55(9): E526. DOI:10.3390/medicina55090526 |
| [4] |
王雪, 郭传影, 王侠, 等. 基于TCGA数据集分析CSTF2基因在肝细胞癌中的表达和临床意义[J]. 实用肿瘤杂志, 2021, 36(6): 550-555. DOI:10.13267/j.cnki.syzlzz.2021.108 |
| [5] |
Srivastava A, Shukla V, Tiwari D, et al. Targeted therapy of chronic liver diseases with the inhibitors of angiogenesis[J]. Biomedecine Pharmacother, 2018, 105: 256-266. DOI:10.1016/j.biopha.2018.05.102 |
| [6] |
Morse MA, Sun WJ, Kim R, et al. The role of angiogenesis in hepatocellular carcinoma[J]. Clin Cancer Res, 2019, 25(3): 912-920. DOI:10.1158/1078-0432.CCR-18-1254 |
| [7] |
Ceci C, Atzori MG, Lacal PM, et al. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: experimental evidence in different metastatic cancer models[J]. Int J Mol Sci, 2020, 21(4): 1388. DOI:10.3390/ijms21041388 |
| [8] |
Weinkopff T, Roys H, Bowlin A, et al. Leishmania infection induces macrophage vascular endothelial growth factor A production in an ARNT/HIF-dependent manner[J]. Infect Immun, 2019, 87(11): e00088-e00019. |
| [9] |
Lin CC, Zhou JP, Liu YP, et al. The silencing of Pokemon attenuates the proliferation of hepatocellular carcinoma cells in vitro and in vivo by inhibiting the PI3K/Akt pathway[J]. PLoS One, 2012, 7(12): e51916. DOI:10.1371/journal.pone.0051916 |
| [10] |
Zhang YQ, Xiao CX, Lin BY, et al. Silencing of Pokemon enhances caspase-dependent apoptosis via fas- and mitochondria-mediated pathways in hepatocellular carcinoma cells[J]. PLoS One, 2013, 8(7): e68981. DOI:10.1371/journal.pone.0068981 |
| [11] |
Wang L, Zhang MX, Zhang MF, et al. ZBTB7A functioned as an oncogene in colorectal cancer[J]. BMC Gastroenterol, 2020, 20(1): 370. DOI:10.1186/s12876-020-01456-z |
| [12] |
Chen L, Zhong J, Liu JH, et al. Pokemon inhibits transforming growth factor β-Smad4-related cell proliferation arrest in breast cancer through specificity protein 1[J]. J Breast Cancer, 2019, 22(1): 15-28. DOI:10.4048/jbc.2019.22.e11 |
| [13] |
Jiang FQ, Zheng QF, Chang LP, et al. Pro-oncogene pokemon promotes prostate cancer progression by inducing STRN4 expression[J]. J Cancer, 2019, 10(8): 1833-1845. DOI:10.7150/jca.29471 |
| [14] |
Chang K, Do SI, Kim K, et al. Proto-oncogene Pokemon in thyroid cancer: a potential promoter of tumorigenesis in papillary thyroid carcinoma[J]. J Pathol Transl Med, 2021, 55(5): 317-323. DOI:10.4132/jptm.2021.06.28 |
| [15] |
Agrawal A, Yang JH, Murphy RF, et al. Regulation of the p14ARF-Mdm2-p53 pathway: an overview in breast cancer[J]. Exp Mol Pathol, 2006, 81(2): 115-122. DOI:10.1016/j.yexmp.2006.07.001 |
| [16] |
Zhu XS, Dai YC, Chen ZX, et al. Knockdown of Pokemon protein expression inhibits hepatocellular carcinoma cell proliferation by suppression of AKT activity[J]. Oncol Res, 2013, 20(8): 377-381. DOI:10.3727/096504013X13657689383012 |
| [17] |
Choi WI, Jeon BN, Yun CO, et al. Proto-oncogene FBI-1 represses transcription of p21CIP1 by inhibition of transcription activation by p53 and Sp1[J]. J Biol Chem, 2009, 284(19): 12633-12644. DOI:10.1074/jbc.M809794200 |
| [18] |
Melincovici CS, Boşca AB, Şuşman S, et al. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis[J]. Rom J Morphol Embryol, 2018, 59(2): 455-467. |
| [19] |
Choi SB, Han HJ, Kim WB, et al. VEGF overexpression predicts poor survival in hepatocellular carcinoma[J]. Open Med (Wars), 2017, 12: 430-439. DOI:10.1515/med-2017-0061 |
| [20] |
Xiong XX, Qiu XY, Hu DX, et al. Advances in hypoxia-mediated mechanisms in hepatocellular carcinoma[J]. Mol Pharmacol, 2017, 92(3): 246-255. DOI:10.1124/mol.116.107706 |
| [21] |
Liu F, Luo LM, Wei YG, et al. Association of VEGFA polymorphisms with susceptibility and clinical outcome of hepatocellular carcinoma in a Chinese Han population[J]. Oncotarget, 2017, 8(10): 16488-16497. DOI:10.18632/oncotarget.14870 |
| [22] |
Zhang L, Wang JN, Tang JM, et al. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells[J]. Mol Biol Rep, 2012, 39(5): 5085-5093. DOI:10.1007/s11033-011-1304-2 |
| [23] |
Bupathi M, Kaseb A, Janku F. Angiopoietin 2 as a therapeutic target in hepatocellular carcinoma treatment: current perspectives[J]. Onco Targets Ther, 2014, 7: 1927-1932. |
| [24] |
Mao CS, Yin H, Ning HB, et al. Levels of HBx, VEGF, and CEACAM1 in HBV-related hepatocellular carcinoma and their correlation with cancer prognosis[J]. Eur Rev Med Pharmacol Sci, 2017, 21(17): 3827-3833. |
| [25] |
都亚薇, 张宁宁, 陆伟. 肝癌免疫治疗的研究现状及展望[J]. 实用肿瘤杂志, 2021, 36(5): 393-398. |
| [26] |
孙瑾瑜, 刘亨晶, 汪泳, 等. ABCB1与肝细胞癌多药耐药的研究进展[J]. 实用肿瘤杂志, 2020, 35(6): 574-578. DOI:10.13267/j.cnki.syzlzz.2020.06.019 |
 2022, Vol. 37
2022, Vol. 37


