文章信息
- 夏浩明, 林鑫
- 肺癌相关转录本1在恶性肿瘤中的研究进展
- 实用肿瘤杂志, 2021, 36(5): 475-481
-
通信作者
- 夏浩明,E-mail:haomingxia@hotmail.com
-
文章历史
- 收稿日期:2020-05-01
2. 哈尔滨医科大学附属第二医院脊柱外科,黑龙江 哈尔滨 150086
真核细胞生物基因组由复杂庞大的DNA序列构成,其中约93%能被转录为RNA,仅2%被翻译成约20 000种蛋白转录产物,余下98%均为编码能力极低或无编码功能的非编码RNA[1-3]。根据长度的不同,ncRNA可分为小非编码RNA和长链非编码RNA(long non-coding RNA,lncRNA),后者碱基组成为200~100 000 nt不等[4]。近年研究发现,lncRNA可参与X染色体沉默、基因组印迹、转录激活和核内运输等多种重要的调控过程,成为机体病理生理过程中不可替代的调控位点[5-9]。肺癌相关转录本1(lung cancer associated transcript 1,LUCAT1)在多种肿瘤中异常高表达,并对肿瘤的发生和发展起到关键调控作用,有望作为肿瘤诊断及患者预后评估的生物标志物和治疗靶点,为肿瘤治疗提供新思路。本文就LUCAT1相关的研究报道作一概述,总结其在肿瘤中的生物学功能和潜在分子机制。
1 LUCAT1概述LUCAT1基因定位于人体5号染色体长臂1区4带(5q14.3),全长890 nt且存在6个外显子。最初在肺癌细胞株中利用RNA测序发现并通过实验证实其在吸烟人群气道上皮细胞和肺癌细胞中异常高表达,因为起初被命名为Smoke and Cancer Associated LncRNA1(SCAL1)[10]。随后大量研究表明,LUCAT1参与调控动脉瘤形成过程中平滑肌细胞凋亡、慢性心力衰竭患者的心肌细胞凋亡和视网膜Müller胶质细胞的免疫反应等病理生理过程[11-13]。此外,LUCAT1在诸多人体恶性肿瘤中呈现异常高表达并可通过内源性海绵吸附、多梳抑制复合物2(polycomb repressive complex 2,PRC2)招募、p53表达抑制和Wnt信号通路激活等多种机制调控肿瘤细胞的增殖、转移侵袭、上皮间质转化及化疗耐药性等恶性生物学行为,对其分子水平的靶向抑制极有可能成为逆转化疗耐药和抗肿瘤分子精准治疗的关键所在。
2 LUCAT1与肿瘤 2.1 肾透明细胞癌免疫细胞释放趋化因子可调控肿瘤细胞中lncRNA的表达。研究发现,肾透明细胞癌中LUCAT1的表达受C-X-C基序趋化因子配体2(C-X-C motif chemokine ligand 2,CXCL2)的正向调控,二者同步高表达且表达水平与肿瘤TMN分期正相关[14]。LUCAT1高表达组患者总生存期(overall survival,OS)与无瘤生存期(disease-free survival,DFS)缩短,提示患者不良预后,表明LUCAT1可作为评估患者生存率的独立判断因素[15]。进一步研究发现,外源性沉默LUCAT1与p-AKT协同表达下调,低表达的p-AKT失去对AKT信号通路下游因子p-糖原合成酶激酶3β(p-glycogen synthase kinase-3β,p-GSK-3β)表达的负向调控,从而实现p-GSK-3β对肿瘤细胞增殖和侵袭转移的抑制效应。LUCAT1通过与PRC2催化亚基zeste同源物2(zeste homolog 2,EZHZ)的增强子结合,介导EZHC对p57启动子的募集作用来实现对p57的表达抑制,从而限制其抑癌效应[16]。研究证实,LUCAT1可通过内源性海绵(sponge)的作用方式抑制miR-495-3p的表达,进而实现miR-495-3p对其下游靶基因SATB同源异形盒1(SATB homeobox 1,SATB1)表达的去抑制,而高表达的SATB1可介导肿瘤细胞的恶性生物学行为[17]。
2.2 肺癌肺癌细胞株中通过RNA测序可识别出LUCAT1的异常高表达[1]。吸烟人群中气道上皮细胞LUCAT1的表达水平高于非吸烟者,同时香烟提取物(cigarette smoke extract,CSE)可提高肿瘤细胞和气道上皮细胞中LUCAT1的表达水平。NF-E2相关因子2(NF-E2-related factor 2,Nrf2)- kelch样ECH相关蛋白1(kelch like ECH associated protein 1,KEAP1)作为重要的信号通路可保护肺上皮细胞免于氧化应激和吸烟损伤。外源性沉默上游调控蛋白KEAP1可实现对NRF2表达的去抑制,高表达的NRF2通过直接与LUCAT1靶向结合实现其表达上调。此外,外源性沉默LUCAT1可增强香烟的细胞毒性,导致肿瘤细胞活性降低。一项对肺癌组织和对应癌旁正常组织的RT-qPCR定量检测研究结果显示,LUCAT1呈上调表达且其表达水平与肿瘤体积和TMN分期相关,同时LUCAT1高表达组患者相较于其低表达组OS缩短[18]。进一步研究发现,肿瘤细胞中LUCAT1和EZH2表达水平正相关,外源性沉默LUCAT1可通过抑制EZH2与p21/p57启动子的结合以及dimethylation of lysine 27 on histone 3(H3K27me3)对p21/p57启动子的二甲基化水平实现二者的高表达。LUCAT1可通过促进胰岛素样生长因子2(insulin like growth factor 2,IGF2)的表达介导非小细胞肺癌的顺铂耐药性[19]。PM2.5可能导致特定位点的DNA甲基化和组蛋白修饰,从而诱导肿瘤的发生。DNA甲基化酶(DNA methyl transferase,DNMT)与lncRNA的异常表达和多种癌症密切相关。研究发现,PM2.5可实现支气管上皮细胞中DNMT1的表达下调,进而通过LUCAT1启动子的去甲基化激活其转录,高表达的LUCAT1可引发PM2.5诱导的活性氧(reactive oxygen species,ROS)瀑布,从而实现细胞毒性反应并介导肿瘤发生[20]。
2.3 消化系统肿瘤组织定量结果显示,肝细胞肝癌中LUCAT1为高表达状态,其表达水平与肿瘤的TNM分期存在高度相关性并可对非癌组织及Ⅰ~Ⅳ期肿瘤组织进行区分,同时单变量与多变量分析结果显示,LUCAT1的表达水平可作为患者生存时间的独立判断因素[21]。致癌因子膜联蛋白(annexin A2,ANXA2)可通过与S100钙结合蛋白A10(S100 calcium binding protein A10,S100A10)形成异四聚体复合物AⅡt发挥作用。研究发现,LUCAT1可通过降低ANXA2 Ser-25的磷酸化水平来抑制AⅡt复合体分离,AⅡt可催化纤溶酶原转化为纤溶酶,后者将pro-基质金属蛋白酶(pro-matrix metalloproteinase,pro-MMP)激活为活性MMP;纤溶酶和MMP均可通过促进肿瘤细胞发生侵袭转移与上皮-间充质转化(epithelial-mesenchymal transition,EMT)推进肝癌发展[22]。一项细胞实验在肝母细胞瘤中证实,LUCAT1由信号传导与转录激活因子3(signal transducer and activator of transcription 3,STAT3)激活表达后借助LUCAT1/miR-301b/STAT3调控轴促进肿瘤细胞增殖,从而形成反馈环路发挥癌基因效应[23]。与上述研究结果不同,Gramantieri等[24]的研究结果显示,LUCAT1高表达除可降低肝细胞肝癌复发率并延长复发时间外,还可通过海绵吸附负向调控miR-181d-5p和上调E-cadherin(CDH1)的表达来抑制肿瘤细胞发生增殖、侵袭与EMT。
结直肠癌患者组织的RT-qPCR定量检测显示,肿瘤组织中LUCAT1异常高表达,且其表达水平与TNM分期和肿瘤退缩分级(tumor regression grade,TRG)相关;外源性沉默LUCAT1可抑制肿瘤细胞的增殖和侵袭转移并促进凋亡发生[25-27]。Ubiquitin A-52 residue ribosomal protein fusion product 1(UBA52)作为杂交基因可编码泛素蛋白和核糖体蛋白L40(ribosomal protein L40,RPL40),二者于翻译后迅速分离;泛素蛋白可通过结合RPL40的下游蛋白MDM2加速其降解,表达下调的MDM2失去对其靶基因p53的表达抑制导致p53高表达。进一步研究发现,LUCA1通过结合UBA52诱导其降解,从而破坏RPL50-MDM2-p53通路,释放更多的MDM2以下调p53。此外,该研究还指出,si-LUCAT1所诱导的p53高表达可降低肿瘤细胞的奥沙利铂(oxaliplatin)和5-氟尿嘧啶的耐药性,实现细胞凋亡率的上升[25]。与上述研究结果相似,Huan等[27]通过转录组测序技术(RNA-sequencing,RNA-seq)和裸鼠成瘤实验发现外援性沉默LUCAT1可通过介导DNA损伤、G2/M细胞周期阻滞和凋亡发生在内的多种途径抑制肿瘤细胞增殖。低氧状态下低氧诱导因子1α(hypoxia inducible factor 1α,HIF-1α)可与LUCAT1基因座的低氧反应元件(hypoxia response elements,HREs)特异性结合并诱导其转录。多聚嘧啶结合蛋白1(polypyrimidine tract binding protein 1,PTBP1)作为LUCAT1的下游靶蛋白,其RRM2和RRM34结构域可实现对特定基因(APP、CD44、CLSTN1、MBNL1和ZNF207)mRNA的可变剪接,而上述基因被证实参与调控细胞增殖、周期调控、凋亡发生和DNA损伤;低氧状态和LUCAT1高表达均可促进PTBP1对目标基因的可变剪接从而诱导肿瘤细胞的恶性生物学行为。进一步研究发现,LUCAT1可削弱氟尿嘧啶、喜树碱(camptothecin,CPT)、多柔比星(adriamycin,ADR)和奥沙利铂诱导的DNA损伤和细胞凋亡,敲减LUCAT1可增强奥沙利铂耐药细胞株的化疗效果,表明LUCAT1可作为潜在治疗靶点为结直肠癌治疗提供新思路。
Nai等[28]的研究则阐述了LUCAT1通过海绵吸附负向调控miR-539促进肿瘤细胞增殖并介导胰腺导管腺癌发生和发展的分子机制。胃癌中LUCAT1的表达水平与肿瘤分期存在正相关,其高表达提示不良临床预后。进一步研究发现,LUCAT1的促细胞增殖效应依赖于其作为内源性海绵对miR-134-5p的负向调控,而tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta(YWHAZ)是miR-134-5p所调控的下游靶基因,在体内和体外过表达的miR-134-5p均能在一定程度上逆转LUCAT1的促肿瘤细胞增殖作用[29]。研究发现,LUCAT1在食管鳞状细胞癌组织中异常高表达,且LUCAT1高表达患者生存率低于其低表达患者[30]。外源性转染siRNA-LUCAT1可抑制肿瘤细胞的增殖和侵袭转移能力并诱导细胞凋亡。沉默LUCAT1可增强caspase 3/7的活性并诱导多聚二磷酸腺苷核糖聚合酶[poly(ADP-ribose)polymerase,PARP]和caspase 9的酶解效应,促进p53/Bax表达和EMT的同时抑制Bcl-2/Bcl-XL的表达。进一步实验证实,DNMT1可参与维持DNA的甲基化,LUCAT1可通过下调ubiquitin like with PHD and ring finger domains 1(UHRF1)的表达抑制DNMT1泛素化并阻滞其降解,从而从转录后水平(甲基化/磷酸化/乙酰化/泛素化)正向调控DNMT1的表达,而高表达的DNMT1通过介导DNA的异常甲基化参与肿瘤的发生和发展。
2.4 乳腺癌乳腺上皮细胞、乳腺癌细胞(breast cancer cells,BCCs)和乳腺癌干细胞(breast cancer stem cells,BCSCs)中LUCAT1表达水平呈递增关系。对151例乳腺癌患者组织的RT-qPCR定量检测显示,LUCAT1的高表达与肿瘤体积、淋巴结转移和TNM分期相关,LUCAT1可作为评估患者整体生存的独立判断因素[31]。肿瘤干细胞(cancer stem cells,CSCs)往往呈现特异性CD44+CD24-表型,LUCAT1可通过上调BCCs中CSCs标志物(SOX/Nanog/OCT4)表达水平和CD44+CD24-细胞比实现其干细胞诱导特性。同时成球实验(sphere-formation assay)结果表明,LUCAT1可提高BCSCs的自我更新能力。进一步研究发现,LUCAT1可通过内源性海绵作用负向调控miR-5582-3p的表达,从而实现miR-5582-3p对下游靶基因transcription factor 7 like 2(TCF7L2)表达的去抑制;低表达的miR-5582-3p可进一步诱导细胞核中β-catenin、Wnt1和SOX2表达上调,β-catenin可启动Wnt信号通路激活下游反应并介导BCCs的类干细胞性以及维持BCSCs的干细胞活性。研究证实,LUCAT1可通过LUCAT1/miR-7-5p/SOX2调控轴上调N-cadherin、MMP-2、MMP-9和下调E-cadherin的表达来促进肿瘤细胞发生转移侵袭与EMT[32]。Mou等[33]也得到相似的研究结果,三阴性乳腺癌组织与肿瘤细胞内LUCAT1为上调表达并且其高表达与患者不良预后密切相关,外源性沉默LUCAT1能够抑制肿瘤细胞的增殖和EMT,诱导细胞周期阻滞并促进凋亡发生。同时该研究还证实,LUCAT1的致癌效应依赖于其作为内源性海绵对miR-5702的负向调控。
2.5 泌尿生殖系统肿瘤RT-qPCR对子宫颈癌组织中LUCAT1表达的检测结果显示,LUCAT1呈异常上调表达并且其表达水平与肿瘤的病理分级、国际妇产科联盟(International Federation of Gynecology and Obstetrics,FIGO)分期、淋巴结转移和子宫颈浸润深度存在相关性;LUCAT1高表达组患者的OS低于其低表达组患者,多变量回归分析(multivariate Cox regression analysis)结果显示,LUCAT1的表达水平可作为子宫颈癌患者OS的独立预测因子。Sp1转录因子(Sp1 transcription factor,SP1)作为上游分子通过结合LUCAT1启动子促进其高表达,LUCAT1可作为竞争性内源RNA(competing endogenous RNA,ceRNA)通过抑制miR-181a诱导肿瘤细胞的恶性生物学行为[34]。Wang等[35]的研究则揭示metastasis associated 1(MTA1)作为LUCAT1下游靶点调控子宫颈癌发生和发展的潜在机制。与上述研究结果类似,Yang等[36]证实,LUCAT1通过负向调控miR-199b-5p的表达抑制子宫颈癌细胞的增殖和侵袭转移并促进凋亡发生。组织定量结果显示,卵巢癌中LUCAT1为高表达状态,外源性沉默LUCAT1可抑制肿瘤细胞的增殖和转移侵袭并诱导细胞凋亡;miR-199a-5p与LUCAT1 3’-UTR结合后对其表达的靶向抑制介导上述效应的发生[37]。
Liu等[38]的研究除证实LUCAT1在前列腺癌组织中异常高表达外,还发现其可通过抑制KiSS-1转移抑制因子(KiSS-1 metastasis suppressor,KISS1)蛋白的表达促进肿瘤细胞的侵袭转移。Cao等[39]利用高通量测序(high-throughput sequencing)发现,高分期膀胱癌组织较正常膀胱组织差异表达269 lncRNAs,较低分期膀胱癌差异表达252 lncRNAs,通过筛选以上两组重叠且表达变化方向相同的lncRNA发现LUCAT1呈现异常高表达,同时基因本体(gene ontology,GO)和信号通路分析(pathway analyses)结果显示,LUCAT1参与细胞转移、细胞黏附过程及Ras信号通路调控。该研究还指出,LUCAT1的表达水平与膀胱癌的临床进展存在正相关,并在诊断膀胱乳头状癌(papillary bladder cancer)和膀胱非乳头状癌(non- papillary bladder cancer)方面具有重要意义。
2.6 骨肉瘤研究发现,甲氨蝶呤(methotrexate)以时间和剂量依赖性方式诱导LUCAT1在甲氨蝶呤耐药骨肉瘤细胞株中表达上调,体内和体外实验证实外源性沉默LUCAT1可在抑制耐药相关基因(MDR1/MRP5/LRP1)表达的同时拮抗肿瘤细胞的增殖和侵袭转移效应,并缩小体内裸鼠移植瘤的体积[40]。进一步研究发现,LUCAT1通过内源性海绵作用抑制miR-200c的表达,实现对miR-200c下游靶点ATP binding cassette subfamily B member 1(ABCB1)表达的去抑制,而ABCB1作为肿瘤耐药相关蛋白诱导肿瘤细胞产生甲氨蝶呤耐药性。
2.7 其他肿瘤LUCAT1在口腔鳞癌组织中表达异常上调,外源性沉默LUCAT1能够抑制肿瘤细胞的增殖和侵袭转移能力[41]。通过线性相关分析(linear correlation analysis)显示,增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)与LUCAT1表达呈正相关[41]。组织定量结果显示,胶质瘤中LUCAT1为高表达状态,且其表达水平与肿瘤分期呈正相关;LUCAT1可通过海绵吸附作用负向调控miR-375从而实现对肿瘤细胞增殖和转移的诱导效应[42]。研究发现,甲状腺乳头癌中LUCAT1的高表达与肿瘤分期呈正相关[43]。外源性沉默LUCAT1可抑制肿瘤细胞的增殖和侵袭转移能力并诱发细胞周期阻滞与细胞凋亡。进一步研究发现,LUCAT1沉默的肿瘤细胞中caspase 8表达上调,caspase 8通过下游因子caspase 3/6/7间接激活核靶点Lamin A/C和PARP从而诱导凋亡发生;同时siRNA-LUCAT1可通过下游高表达靶点(p21/p57/p53/BAX)和低表达靶点(EZH2/DNMT1/CDK1/HDAC1/NRF2)改变肿瘤细胞的生物学行为,调控肿瘤的发生和发展。体外和体内实验证实,外源性沉默LUCAT1可抑制脉络膜黑色素瘤(choroidal melanoma,CM)细胞的增殖和侵袭转移能力,降低裸鼠移植瘤的体积和重量从而减缓肿瘤的生长进程[44]。进一步研究发现,E74 like ETS transcription factor 1(ELF1)作为上游转录因子通过与LUCAT1启动子结合激活其转录,高表达的LUCAT1借助LUCAT1/miR-514a/b-3p/ RBX1调控轴实现对CM的诱导效应。
3 结语lncRNA起初被认为是基因组转录的“噪声”,是RNA聚合酶Ⅱ转录的副产物,并不具有任何生物学功能。随着高通量测序技术的推进,大量lncRNA的发现及其在多种肿瘤中的异常表达使得lncRNA在肿瘤调控中的作用机制成为研究的热点。LUCAT1这一起初从肺癌细胞株中发现的lncRNA于多种肿瘤组织中高表达。其可通过内源性海绵吸附、PRC2招募、p53表达抑制和Wnt信号通路激活等多重机制在表观遗传、转录及转录后等不同水平实现不同组织的癌性衍化(图 1)。对LUCAT1组织及血清表达水平的检测有望为癌症患者的术前诊断、肿瘤分期及临床预后提供参考,同时对其分子水平的研究使得LUCAT1有望成为日后癌症治疗的有效靶点。然而目前肿瘤中LUCAT1的调控作用研究尚处于萌芽阶段,对其在肿瘤中所呈现的不同表达状态、详细的下游基因表达的分子调控机制以及肿瘤内LUCAT1失调控的上游分子事件等问题均有待后续的深入性研究。
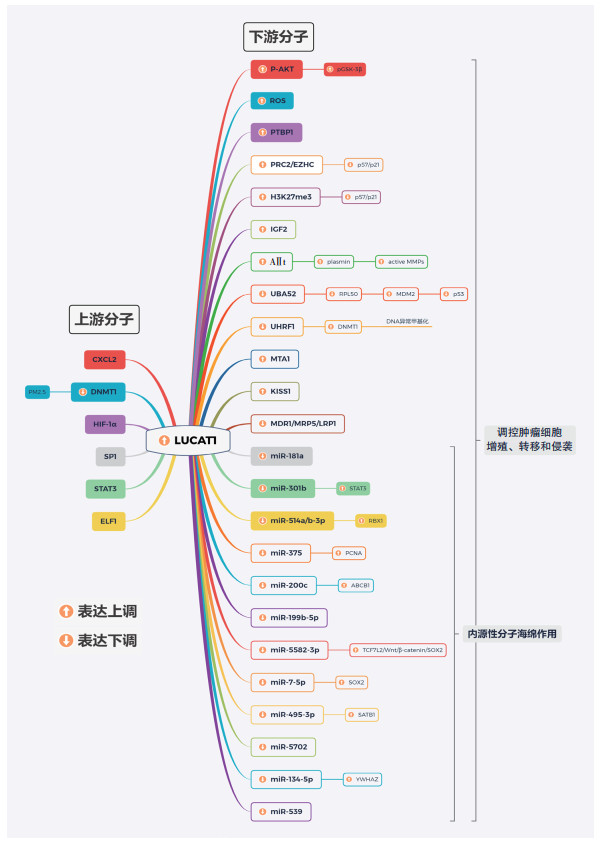
|
| 注 CXCL2:C-X-C基序趋化因子配体2(C-X-C motif chemokine ligand 2);DNMT1:DNA甲基化酶1(DNA methyltransferase 1);HIF- 1α:低氧诱导因子1α(hypoxia inducible factor 1α);SP1:Sp1转录因子(Sp1 transcription factor);STAT3:信号传导与转录激活因子3(signal transducer and activator of transcription 3);ELF1:E74样ETS转录因子1(E74 like ETS transcription factor 1);LUCAT1:肺癌相关转录本1(lung cancer associated transcript 1);AKT:丝氨酸/ 苏氨酸激酶(AKT serine/threonine kinase);GSK-3β:糖原合成酶激酶3β(glycogen synthase kinase-3β);ROS:活性氧(reactive oxygen species);PTBP1:多聚嘧啶结合蛋白1(polypyrimidine tract binding protein 1);PRC2:多梳抑制复合体2(polycomb repressive complex 2);EZHC:zeste同源物2(zeste homolog 2);IGF2:促进胰岛素样生长因子2(insulin like growth factor 2);MMP:基质金属蛋白酶(matrix metalloproteinase);UBA52:ubiquitin A-52 residue ribosomal protein fusion product 1;RPL40:核糖体蛋白L40(ribosomal protein L40);MDM2:MDM2 proto-oncogene;UHRF1:ubiquitin like with PHD and ring finger domains 1;MTA1:metastasis associated 1;KISS1:KiSS-1转移抑制因子(KiSS-1 metastasis suppressor);MDR1:多药耐药基因1(multidrug resistance 1);MRP5:多药耐药相关蛋白5(multidrug resistance-associated protein 5);RBX1:ring-box 1;PCNA:增殖细胞核抗原(proliferating cell nuclear antigen);ABCB1:ATP binding cassette subfamily B member 1;TCF7L2:transcription factor 7 like 2;SOX2:SRY盒转录因子2(SRY-box transcription factor 2);SATB1:SATB同源异形盒1(SATB homeobox 1);YWHAZ:tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta 图 1 恶性肿瘤中LUCAT1调控作用的分子机制 |
| [1] |
Cremer S, Michalik KM, Fischer A, et al. Hematopoietic deficiency of the long noncoding RNA MALAT1 promotes atherosclerosis and plaque inflammation[J]. Circulation, 2019, 139(10): 1320-1334. DOI:10.1161/CIRCULATIONAHA.117.029015 |
| [2] |
Ling H, Fabbri M, Calin GA. MicroRNAs and other noncoding RNAs as targets for anticancer drug development[J]. Nat Rev Drug Discov, 2013, 12(11): 847-865. |
| [3] |
Fu XD. Non-coding RNA: a new frontier in regulatory biology[J]. Natl Sci Rev, 2014, 1(2): 190-204. DOI:10.1093/nsr/nwu008 |
| [4] |
Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms[J]. Cell, 2013, 154(1): 26-46. DOI:10.1016/j.cell.2013.06.020 |
| [5] |
宋文渊, 武金才, 吴雷, 等. 血清lncRNA-PVT1在肝细胞癌诊断及预后中的意义[J]. 实用肿瘤杂志, 2020, 35(4): 317-321. |
| [6] |
Cipolla GA, de Oliveira JC, Salviano-Silva A, et al. Long non-coding RNAs in multifactorial diseases: another layer of complexity[J]. Noncoding RNA, 2018, 4(2): 13. |
| [7] |
Hu G, Niu F, Humburg BA, et al. Molecular mechanisms of long noncoding RNAs and their role in disease pathogenesis[J]. Oncotarget, 2018, 9(26): 18648-18663. DOI:10.18632/oncotarget.24307 |
| [8] |
Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer[J]. Nat Rev Genet, 2016, 17(12): 719-732. |
| [9] |
Giordano S, Columbano A. MicroRNAs: new tools for diagnosis, prognosis, and therapy in hepatocellular carcinoma?[J]. Hepatology, 2013, 57(2): 840-847. DOI:10.1002/hep.26095 |
| [10] |
Thai P, Statt S, Chen CH, et al. Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines[J]. Am J Respir Cell Mol Biol, 2013, 49(2): 204-211. DOI:10.1165/rcmb.2013-0159RC |
| [11] |
Xia Q, Zhang L, Yan H, et al. LUCAT1 contributes to MYRF-dependent smooth muscle cell apoptosis and may facilitate aneurysm formation via the sequestration of miR-199a-5p[J]. Cell Biol Int, 2020, 44(3): 755-763. DOI:10.1002/cbin.11270 |
| [12] |
Li T, Qian D, Guoyan J, et al. Downregulated long noncoding RNA LUCAT1 inhibited proliferation and promoted apoptosis of cardiomyocyte via miR-612/HOXA13 pathway in chronic heart failure[J]. Eur Rev Med Pharmacol Sci, 2020, 24(1): 385-395. |
| [13] |
Rochet E, Appukuttan B, Ma Y, et al. Expression of long non-coding RNAs by human retinal Müller glial cells infected with clonal and exotic virulent toxoplasma gondii[J]. Noncoding RNA, 2019, 5(4): 48. |
| [14] |
Zheng Z, Zhao F, Zhu D, et al. Long non-coding RNA LUCAT1 promotes proliferation and invasion in clear cell renal cell carcinoma through AKT/GSK-3β signaling pathway[J]. Cell Physiol Biochem, 2018, 48(3): 891-904. DOI:10.1159/000491957 |
| [15] |
Wang Y, Li Z, Li W, et al. Prognostic significance of long non-coding RNAs in clear cell renal cell carcinoma: A meta-analysis[J]. Medicine (Baltimore), 2019, 98(40): e17276. DOI:10.1097/MD.0000000000017276 |
| [16] |
Xiao H, Bao L, Xiao W, et al. Long non-coding RNA Lucat1 is a poor prognostic factor and demonstrates malignant biological behavior in clear cell renal cell carcinoma[J]. Oncotarget, 2017, 8(69): 113622-113634. DOI:10.18632/oncotarget.21185 |
| [17] |
Wang LN, Zhu XQ, Song XS, et al. Long noncoding RNA lung cancer associated transcript 1 promotes proliferation and invasion of clear cell renal cell carcinoma cells by negatively regulating miR-495-3p[J]. J Cell Biochem, 2018, 119(9): 7599-7609. DOI:10.1002/jcb.27099 |
| [18] |
Sun Y, Jin SD, Zhu Q, et al. Long non-coding RNA LUCAT1 is associated with poor prognosis in human non-small lung cancer and regulates cell proliferation via epigenetically repressing p21 and p57 expression[J]. Oncotarget, 2017, 8(17): 28297-28311. DOI:10.18632/oncotarget.16044 |
| [19] |
Wang W, Dong ML, Zhang W, et al. Long noncoding LUCAT1 promotes cisplatin resistance of non-small cell lung cancer by promoting IGF-2[J]. Eur Rev Med Pharmacol Sci, 2019, 23(12): 5229-5234. |
| [20] |
Ling XX, Zhang HQ, Liu JX, et al. LncRNA LUCAT1 activation mediated by the down-regulation of DNMT1 is involved in cell apoptosis induced by PM2.5[J]. Biomed Environ Sci, 2018, 31(8): 608-612. |
| [21] |
Jiao Y, Li Y, Ji B, et al. Clinical value of lncRNA LUCAT1 expression in liver cancer and its potential pathways[J]. J Gastrointestin Liver Dis, 2019, 28(4): 439-447. DOI:10.15403/jgld-356 |
| [22] |
Lou Y, Yu Y, Xu X, et al. Long non-coding RNA LUCAT1 promotes tumourigenesis by inhibiting ANXA2 phosphorylation in hepatocellular carcinoma[J]. J Cell Mol Med, 2019, 23(3): 1873-1884. DOI:10.1111/jcmm.14088 |
| [23] |
Wang X, Guo S, Zhao R, et al. STAT3-activated long non-coding RNA lung cancer associated transcript 1 drives cell proliferation, migration, and invasion in hepatoblastoma through regulation of the miR-301b/STAT3 Axis[J]. Hum Gene Ther, 2019, 30(6): 702-713. DOI:10.1089/hum.2018.146 |
| [24] |
Gramantieri L, Baglioni M, Fornari F, et al. LncRNAs as novel players in hepatocellular carcinoma recurrence[J]. Oncotarget, 2018, 9(80): 35085-35099. DOI:10.18632/oncotarget.26202 |
| [25] |
Zhou Q, Hou Z, Zuo S, et al. LUCAT1 promotes colorectal cancer tumorigenesis by targeting the ribosomal protein L40-MDM2-p53 pathway through binding with UBA52[J]. Cancer Sci, 2019, 110(4): 1194-1207. DOI:10.1111/cas.13951 |
| [26] |
Chen Y, Yu X, Xu Y, et al. Identification of dysregulated lncRNAs profiling and metastasis-associated lncRNAs in colorectal cancer by genome-wide analysis[J]. Cancer Med, 2017, 6(10): 2321-2330. DOI:10.1002/cam4.1168 |
| [27] |
Huan L, Guo T, Wu Y, et al. Hypoxia induced LUCAT1/ PTBP1 axis modulates cancer cell viability and chemotherapy response[J]. Mol Cancer, 2020, 19(1): 11. DOI:10.1186/s12943-019-1122-z |
| [28] |
Nai Y, Pan C, Hu X, et al. LncRNA LUCAT1 contributes to cell proliferation and migration in human pancreatic ductal adenocarcinoma via sponging miR-539[J]. Cancer Med, 2020, 9(2): 757-767. |
| [29] |
Chi J, Liu T, Shi C, et al. Long non-coding RNA LUCAT1 promotes proliferation and invasion in gastric cancer by regulating miR-134-5p/YWHAZ axis[J]. Biomed Pharmacother, 2019, 118(3): 109201. |
| [30] |
Yoon JH, You BH, Park CH, et al. The long noncoding RNA LUCAT1 promotes tumorigenesis by controlling ubiquitination and stability of DNA methyltransferase 1 in esophageal squamous cell carcinoma[J]. Cancer Lett, 2018, 417(1): 47-57. |
| [31] |
Zheng A, Song X, Zhang L, et al. Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis regulates breast cancer stemness via Wnt/β-catenin pathway[J]. J Exp Clin Cancer Res, 2019, 38(1): 305. |
| [32] |
Li YL, Wang XM, Qiao GD, et al. Up-regulated lnc-lung cancer associated transcript 1 enhances cell migration and invasion in breast cancer progression[J]. Biochem Biophys Res Commun, 2020, 521(2): 271-278. |
| [33] |
Mou E, Wang H. LncRNA LUCAT1 facilitates tumorigenesis and metastasis of triple-negative breast cancer through modulating miR-5702[J]. Biosci Rep, 2019, 39(9): BSR20190489. |
| [34] |
Zhang L, Liu SK, Song L, et al. SP1-induced up-regulation of lncRNA LUCAT1 promotes proliferation, migration and invasion of cervical cancer by sponging miR-181a[J]. Artif Cells Nanomed Biotechnol, 2019, 47(1): 556-564. |
| [35] |
Wang AH, Zhao JM, Du J, et al. Long noncoding RNA LUCAT1 promotes cervical cancer cell proliferation and invasion by upregulating MTA1[J]. Eur Rev Med Pharmacol Sci, 2019, 23(16): 6824-6829. |
| [36] |
Yang T, Xia S. Study of the biological function of LncRNA LUCAT1 on cervical cancer cells by targeting miR-199b- 5p[J]. Biosci Rep, 2020, 40(4): BSR20200422. |
| [37] |
Liu HZ, Liu GY, Pang WW, et al. LncRNA LUCAT1 promotes proliferation of ovarian cancer cells by regulating miR-199a-5p expression[J]. Eur Rev Med Pharmacol Sci, 2020, 24(4): 1682-1687. |
| [38] |
Liu C, Wang L, Li YW, et al. Long noncoding RNA LUCAT1 promotes migration and invasion of prostate cancer cells by inhibiting KISS1 expression[J]. Eur Rev Med Pharmacol Sci, 2019, 23(8): 3277-3283. |
| [39] |
Cao YP, Zhou J, Li WJ, et al. Long non-coding RNA expression profiles for the characterization of different bladder cancer grade[J]. Cell Physiol Biochem, 2018, 50(3): 1154-1163. |
| [40] |
Han Z, Shi L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ ABCB1 axis[J]. Biochem Biophys Res Commun, 2018, 495(1): 947-953. |
| [41] |
Kong Y, Feng Y, Xiao YY, et al. LncRNA LUCAT1 promotes rowth, migration, and invasion of oral squamous cell carcinoma by upregulating PCNA[J]. Eur Rev Med Pharmacol Sci, 2019, 23(11): 4770-4776. |
| [42] |
Gao YS, Liu XZ, Zhang YG, et al. Knockdown of long noncoding RNA LUCAT1 inhibits cell viability and invasion by regulating miR-375 in glioma[J]. Oncol Res, 2018, 26(2): 307-313. |
| [43] |
Luzón-Toro B, Fernández RM, Martos-Martínez JM, et al. LncRNA LUCAT1 as a novel prognostic biomarker for patients with papillary thyroid cancer[J]. Sci Rep, 2019, 9(1): 14374. |
| [44] |
Wang L, Tang D, Wu T, et al. ELF1-mediated LUCAT1 promotes choroidal melanoma by modulating RBX1 expression[J]. Cancer Med, 2020, 9(6): 2160-2170. |
 2021, Vol. 36
2021, Vol. 36


