2. 水域环境生态工程研究中心, 上海 201306;
3. 上海海洋大学 水产与生命学院, 上海 201306
全氟烷基化合物(Perfluorinated Organic Compounds,PFCs)是一类持久性有机污染物,其降解产物主要为全氟辛烷磺酸(perfluoro-rooctane sulfonate,PFOS)和全氟辛酸(perfluorooctanoic acid,PFOA)[1]。PFOS的疏水疏油性使其难以降解并可沿食物链富集放大,且具有多种毒性[2-4]。研究者普遍认为膳食摄入是PFOS进入人体的主要途径,水产品[5-6]、蛋肉类[7]、乳制品[8-9]、果蔬[10]中检测到PFOS,且在自来水[11-12]、湖泊[13-14]、湿地[15-16]、地下水及近岸河口[17-18]区域中均检测到PFOS。
目前,全氟化合物的定量检测方法有气质联用(GC-MS)[19]、液相色谱-质谱联用(LC-MS)、液相色谱-串联质谱联用(LC-MS/MS)[20-21]等。PFOS稳定性强,采用GC-MS时需将其衍生化,但衍生化步骤繁琐,易产生外部干扰[19]。HPLC-MS/MS的多反应监测模式,可减少背景干扰,且适合环境中痕量物质的检测,经过液液萃取法、索式提取法、超声提取法等[22-24]前处理,并结合固相萃取技术,可取得良好的检测结果。
水中PFOS含量检测,在前处理时一般采用离子交换WAX柱、反相萃取柱HLB柱富集净化。SPELTINI等[25]利用SPE-UPLC-ESI-MS检测地表水中PFOS与PFOA含量,通过制备多壁碳纳米管吸附剂作为SPE吸附剂,水样经5 mL甲醇、5 mL水活化后,抽真空10 min,再用6 mL甲醇洗脱,河水加标回收率仅为70.0%~88.5%;杨文龙等[26]利用超高效液相色谱串联四极杆质谱检测水中PFOS含量,水样前处理时选用WAX柱,经过0.5%氨水甲醇溶液、甲醇、超纯水活化,再分别用8 mL超纯水、8 mL乙酸铵溶液(pH=4)淋洗,最后洗脱时先用8 mL甲醇溶液淋洗,弃掉淋洗液,6 mL 0.5%氨水甲醇溶液洗脱小柱;黄东仁等[27]利用液相色谱-串联质谱测定近岸及河口中全氟化合物,选用C18固相萃取小柱对水样进行富集净化,C18柱经6 mL正己烷、丙酮、甲醇、超纯水活化,再用15 mL甲醇-乙酸乙酯洗脱,氮吹复溶。杨文龙和黄东仁所建立的实验方法都取得了较好的检测结果,但水样前处理步骤较繁琐。本文在此基础上,对水中PFOS前处理和上机检测的主要操作步骤、溶剂用量、检测时间等继续优化,为水中PFOS检测提供更为便捷的操作流程。
1 材料与方法 1.1 仪器与试剂实验仪器包括:液相色谱-串联四级杆质谱联用仪(Waters_TQS);ACQΜITY ΜPLC BEH C18 Column色谱柱(2.1 mm×100 mm,1.7 μm);CORTECS ΜPLC T3 Column色谱柱(4.6 mm×100 mm,2.7 μm);Poly-Sery HLB固相萃取柱(6 cm,500 mg);BOND LC-C18固相萃取柱(3 mL,500 mg);0.22 μm有机尼龙滤膜、0.22 μm亲水型PTFE滤膜、0.22 μm聚丙烯微孔滤膜(上海安谱);eppendor离心机(Centrifuge 5810R);固相萃取仪(Agilent SampliQ 20);恒温混匀仪(杭州瑞诚仪器有限公司,TS100);制水机(PΜRELAB Option0)。
实验试剂包括:PFOS标准溶液(100 mg/mL Sigma);13C4-PFOS标准溶液(100 mg/mL Wellington);HPLC-甲醇(上海安谱);HPLC-乙腈(上海安谱);HPLC-水(Sigma-Aldrich公司);甲酸铵、甲酸(Sigma-Aldrich公司)。
1.2 PFOS检测方法优化 1.2.1 上机检测条件优化取10 μg/L的PFOS标准溶液1 mL,以水-甲醇、水-乙腈为流动相,分别加入0.1%甲酸、0.1%甲酸铵、5 mmol/L NH4OH溶液以调节流动相pH分别至酸性、中性和碱性,HPLC-MS上机检测,选择峰型好、响应值高的作为流动相。
确定流动相后,分别用BEH C18和CORTECS ΜPLC T3色谱柱对10 μg/L的PFOS标准溶液进行分离,确定分离效果好的色谱柱。
定量离子取10 μg/L的PFOS标准溶液在负离子模式下进行扫描,目标离子监测采用多反应模式,以峰面积响应值较高的碎片离子作为实验定量离子。
1.2.2 水样前处理条件优化为避免萃取柱堵塞,需用滤膜对水样进行初过滤以除去杂质,但又不能影响水中PFOS定量分析。分别取10 μg/L的PFOS溶液1 mL,用0.22 μm的亲水PTFE型、有机相尼龙型和PP聚丙烯型3种滤膜过滤后上机检测(n=3),选择结果最优者进行水样初过滤。
分别用5%、10%、20%、30%、40%、50%的甲醇溶液配制质量浓度为10 μg/L的PFOS溶液上机测试(n=3),选择加标回收率满足要求的甲醇助溶剂浓度。
用30%的甲醇溶液作助溶剂配制0.1 μg/L的PFOS溶液200 mL,分别选用HLB萃取柱、C18柱和SAX柱对PFOS溶液进行富集处理。选择PFOS回收率高、操作步骤简单、溶剂用量较少的作为固相萃取小柱。
用30%甲醇配制0.1 μg/L的PFOS溶液200 mL,将溶液过萃取柱富集后,再分别用3、4 mL纯甲醇溶液洗脱吸附于HLB柱上的PFOS(n=3),以回收率高者作为更佳的洗脱液体积。
1.3 水样中PFOS的测定方法水样取自某校园内湖水、上海市郊区某虾类养殖塘进水和排水,采集水样1 L,水样贮存于聚丙烯瓶中,运至实验室后4℃保存,并在一周内完成样品处理与测试。
量取200 mL水样过0.22 μm PP滤膜至玻璃烧杯中,加入10 ng内标,摇匀备用;依次用5 mL甲醇、5 mL超纯水重力活化HLB固相萃取柱,流速约2~3滴/s;待活化结束后,打开真空泵,即刻将水样以3 mL/min的速度过柱富集;待富集结束后,将阀门开至最大,用3 mL 10%甲醇水溶液快速淋洗小柱以清除表面杂质;抽真空1 h;关闭真空泵,用4 mL纯甲醇溶液重力洗脱萃取柱,将洗脱液收集至聚丙烯离心管中;取1 mL洗脱液于色谱进样瓶中,用高效液相色质-串联四极杆联用仪上机测定。
色谱柱为ACQΜITY ΜPLC BEH C18 Column;柱温:38 ℃;进样体积:2.0 μL;总流速:0.3 mL/min;流动相A:0.1%甲酸,流动相B:甲醇,梯度洗脱条件:0~1 min,30% B;1.1 min,100% B;1.1~5 min,100% B;5.1 min,30% B;5.1~7 min,30% B。电喷雾离子源(ESI);负离子模式;源温度120 ℃;毛细管电压3.0 kV;干燥N2温度为250 ℃;流速6 L/min;实验采用多反应监测模式(MRM);实验定量离子对PFOS,m/z=499/80。
1.4 质量控制与保证为避免外源性污染,实验所用容器都采用聚丙烯材质,在使用前用超纯水和甲醇交替冲洗2~3次,为了检查残留效应和背景污染,每10个样品运行1次空白溶液,所有空白检测值低于检测限。以已知低浓度样品与空白样品的3倍信噪比为仪器检出限。设置空白对照与标准样品检验实验过程是否有PFOS影响,配制PFOS溶液(n=6)进行重复检测,偏差小于20%,向空白样品添加不同浓度PFOS(n=3),其平均变异系数(CV)为4.05%。
1.5 方法学实验用30%甲醇水溶液配制0.05 μg/L的PFOS溶液200 mL,设7份平行样品,按照1.3节方法测定,计算7次平行测定结果的标准偏差s。以3.14 s作为方法检出限,4倍方法检出限为定量限。
向空白水样中添加PFOS,设置3个不同质量浓度(0.2、2.0和20 μg/L),每个质量浓度3个平行,按照1.3节方法测定,按公式(1)(2)计算加标回收率和相对标准偏差(RSD)。
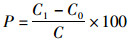 (1)
(1)
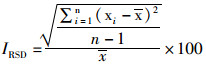 (2)
(2)
式中:P为样品加标回收率,%;C1为加标样品测定值,μg/L;C0为样品背景值,μg/L;C为加样量,μg/L;IRSD为相对标准偏差;n表示样品重复测定次数;x表示n次测量数据的算数平均值。
2 结果与讨论 2.1 仪器条件的优化 2.1.1 流动相选择多数研究者以水-甲醇、水-乙腈为流动相[21, 28]。本文也以水-甲醇、水-乙腈为流动相,同时分别加入0.1%甲酸、0.1%甲酸铵、5 mmol/L NH4OH调节流动相pH。由图 1可知,PFOS溶液在水-甲醇流动相体系下,出峰时间较稳定,峰形光滑、无拖尾且保留时间较长。经内标校正定量后,在水-甲醇体系下,加入0.1%甲酸溶液、0.1%甲酸铵、5 mmol/L NH4OH时,峰面积响应值分别为53 600、51 344、51 280,PFOS含有烷磺酸基团,在酸性条件下可实现较好的分离。最终,实验选择0.1%甲酸水溶液-甲醇溶液为流动相。
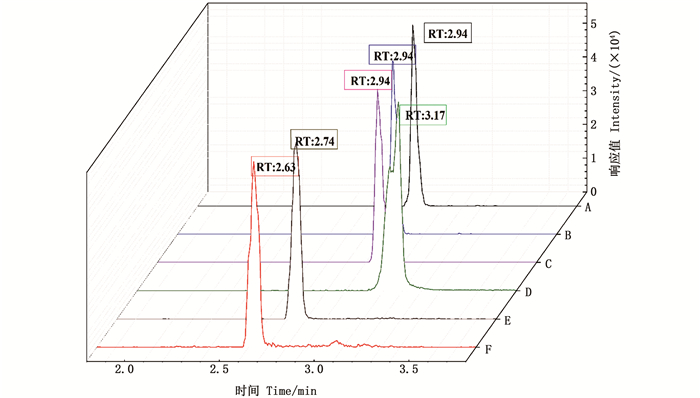
|
A为0.1%甲酸水溶液-甲醇;B为0.1%甲酸铵水溶液-甲醇;C为5 mmol/L氨水-甲醇;D为0.1%甲酸水溶液-乙腈;E为0.1%甲酸铵水溶液-乙腈;F为5 mmol/L氨水-乙腈。 A is 0.1% formic acid aqueous solution-methanol; B is 0.1% ammonium formate aqueous solution-methanol; C is 5 mmol/L ammonia-methanol; D is 0.1% formic acid aqueous solution-acetonitrile; E is 0.1% ammonium formate aqueous solution-acetonitrile; F 5 mmol/L ammonia-acetonitrile. 图 1 流动相的对比 Fig. 1 Comparison of mobile phases |
不同类型色谱柱对PFOS的分离效果存在一定的影响,本实验在流动相体系为0.1%甲酸水溶液-甲醇时,采用BEH C18和CORTECS ΜPLC T3两种色谱柱对浓度为10 μg/L的PFOS标准溶液进行分离,结果如图 2所示。BEH C18色谱柱的分离效果远高于CORTECS ΜPLC T3,色谱峰峰型光滑、无拖尾、保留时间较长、响应值高。
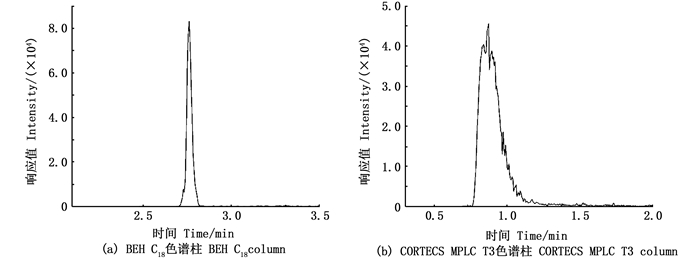
|
图 2 两种色谱柱在PFOS浓度为10 μg/L下的色谱峰 Fig. 2 Chromatographic peaks of C18 and T3 columns at 10 μg/mL of PFOS standard solution |
全氟辛烷磺酸带有-SO3-,因此实验选择在负离子模式下进行扫描,目标离子监测采用多反应模式。CHEN等[29]在对PFOS定量时选择m/z=99,而APARICIO等[30]选择了m/z=80。实验中,在二级扫描时出现两个丰度相对较高的碎片离子(m/z=80、99)。碎片离子m/z=80色谱峰响应值明显高于碎片离子m/z=99(图 3),因此将碎片离子m/z=80作为定量离子。
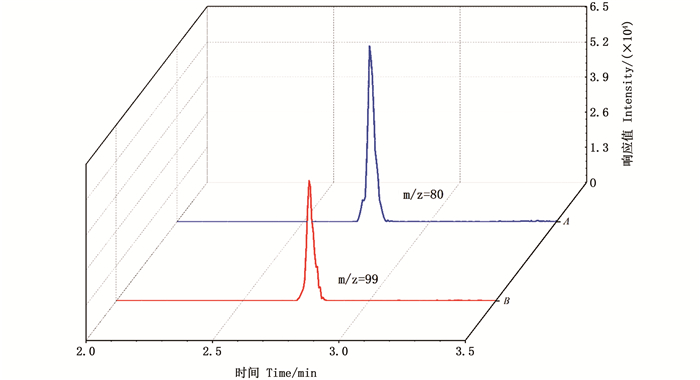
|
图 3 碎片离子m/z(80、99)色谱峰 Fig. 3 Fragment ion m/z (80, 99) chromatographic peak |
10 μg/L的PFOS溶液经有机尼龙型、亲水型和PP滤膜3种滤膜过滤后的检测结果分别为3.91、3.02和9.24 μg/L,即前两种滤膜对PFOS有较强的吸附影响,与董文洪等[31]研究发现“尼龙滤膜对PFOS有较强的吸附性”一致。因此,选择0.22 μm PP滤膜对水样进行预处理。
2.2.2 助溶剂用5%、10%、20%、30%、40%、50%的甲醇溶液配制的PFOS溶液上机检测,甲醇含量为5%~20%,PFOS回收率逐渐增加,当甲醇含量为30%时,PFOS回收率最大,甲醇含量为30%~50%时,PFOS回收率无明显变化(图 4)。为节约溶剂用量,实验选择用30%甲醇溶液做助溶剂。
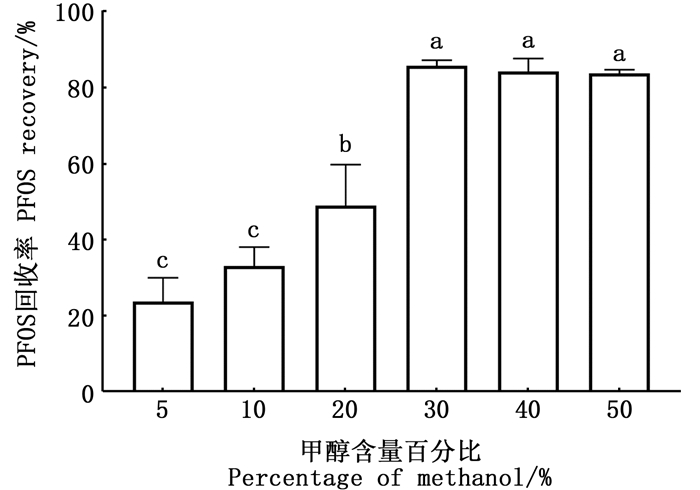
|
不同字母表示差异性显著(P < 0.05)。 Different letters indicate significant differences (P < 0.05). 图 4 不同百分比甲醇助溶剂中10 μg/L的PFOS溶液的回收率 Fig. 4 Recovery rate of 10 μg/L PFOS solution in methanol co-solvent with different percentages |
结果表明,HLB柱、C18柱和SAX柱的加标回收率分别为90.6%、83.1%和29.8%,HLB柱和C18柱对PFOS富集效果较好,而SAX柱富集效果最差。反相萃取HLB固相萃取柱,适用于极性较强的化合物,并且在pH为1~14范围内都非常稳定,PFOS可有效吸附于HLB柱上; 对于SAX柱而言,PFOS属于弱酸性化合物,而SAX柱属于强阴离子萃取柱,在吸附和淋洗过程中,易导致目标物损失。最终,本实验选择Poly-Sery HLB柱作为样品SPE富集净化柱。
2.2.4 SPE洗脱体积用4 mL甲醇洗脱吸附于HLB柱上的PFOS时,PFOS平均回收率为71.76%,而用3 mL甲醇洗脱平均回收率仅为43.3%。推测当洗脱液为3 mL时尚不足以将吸附在固相萃取柱上的PFOS完全洗脱。因此,实验选择体积为4 mL的甲醇溶液为HLB柱洗脱液。王懿等[32]以ACQΜITY ΜPLC BEH C18为色谱分析柱,利用SPE前处理技术,选用HLB(Oasis)萃取柱对水中PFOS富集,用8 mL含0.1%甲酸的甲醇溶液作为洗脱液,其方法加标回收率为81.9%~144.7%。本实验也以ACQΜITY ΜPLC BEH C18为分析柱,选用HLB萃取柱,当洗脱液为4 mL的纯甲醇溶液时,PFOS加标回收率为82.1%~104.5%。
2.3 方法学实验结果 2.3.1 线性范围与检出限配制PFOS标准系列溶液0.01、0.02、0.05、0.1、0.2、0.5、1、2、5、10、20、50 μg/L,并加入内标物。结果如表 1所示,PFOS标准系列溶液在0.01~50 μg/L范围内线性良好,相关系数为0.998,符合GB/T 27417—2017关于相关系数不低于0.99的要求。经计算仪器检出限(ILOD)为7.6 ng/L,方法检出限(MLOD)为0.25 μg/L。
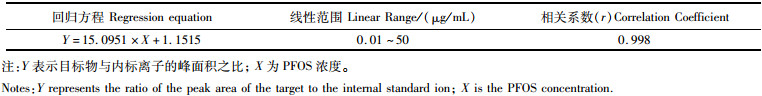
|
表 1 PFOS标准曲线回归方程、线性范围与相关系数 Tab.1 PFOS standard curve regression equation, linear range and correlation coefficient |
用30%甲醇溶液分别配制3组不同浓度(0.2、2.0、20 μg/L)的PFOS溶液200 mL,每组3个平行样品,按1.3节样品前处理后上机测试。实验数据见表 2,3组加标回收率为82.1%~104.5%,相对标准偏差RSD < 5.64%,实验结果符合GB/T 27417—2017[33]“当浓度水平范围 < 0.1 μg/mL时,回收率范围为60%~120%”的要求。
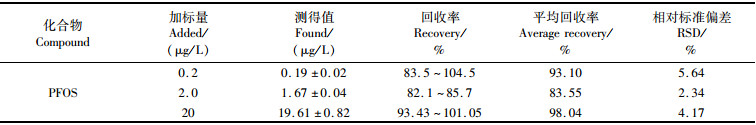
|
表 2 PFOS的方法回收率 Tab.2 Recovery of PFOS |
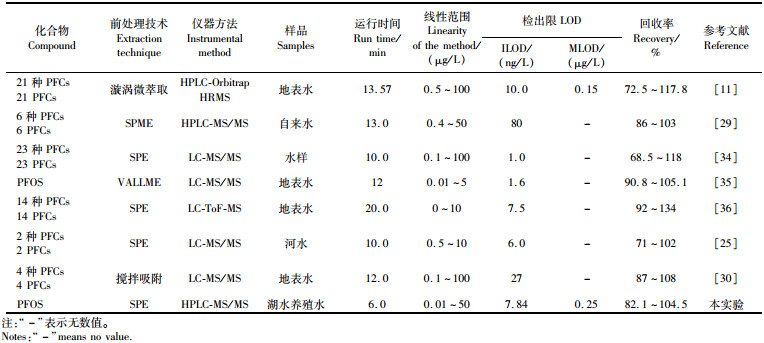
表 3列出了本方法和其他文献中报道的特征数据,与前人的研究相比,本文所建立的方法总体上具有运行时间和溶剂消耗更少,回收率稳定,仪器灵敏度较高、检出限相对较低、前处理操作较简便等优点。
|
表 3 水样中PFOS含量测定方法对比 Tab.3 Comparison of PFOS determination methods in water samples |
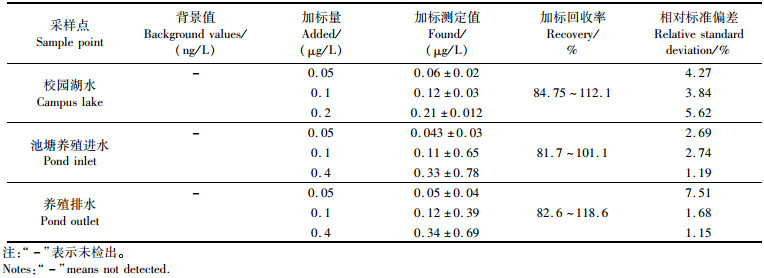
取上海市郊区某虾类养殖场池塘进水、池塘排水以及某校园内湖水样,按照1.3节的方法进行测定,结果列于表 4,校园湖水、虾类养殖场池塘进出水样中PFOS含量均低于检出限。校园湖水加标浓度分别为0.05、0.1、0.2 μg/L,回收率为84.75%~112.1%,RSD < 5.62%;虾池养殖水样加标浓度分别为0.05、0.1、0.4 μg/L,回收率为81.7%~118.6%,RSD < 7.51%。
|
表 4 实际样品测定值及加标回收值 Tab.4 Measured values of actual samples and standard recovery values |
利用反相萃取柱(HLB)对水样进行前处理,结合高效液相色谱-串联四极杆质谱技术建立了水中全氟辛烷磺酸(PFOS)的测定方法。对PFOS的LC-MS-MS分析条件进行优化:确定BEH C18色谱柱、0.1%甲酸溶液-甲醇流动相、m/z=80定量离子为最优仪器条件。用0.22 μm PP滤膜对水样进行初过滤,同时对比HLB、C18、SAX萃取柱对PFOS回收率的影响,选用HLB柱为SPE柱。所建立的高效液相色谱-串联质谱法可有效去除水样的基质干扰,线性范围宽,仪器灵敏度高、回收率和方法重复性良好。
| [1] |
宋彦敏, 周连宁, 郝文龙, 等. 全氟化合物的污染现状及国内外研究进展[J]. 环境工程, 2017, 35(10): 82-86. SONG Y M, ZHOU L N, HAO W L, et al. Pollution status of perfluorinated compounds and research progress at home and abroad[J]. Environmental Engineering, 2017, 35(10): 82-86. |
| [2] |
HUANG J, SUN L, MENNIGEN J A, et al. Developmental toxicity of the novel PFOS alternative OBS in developing zebrafish: An emphasis on cilia disruption[J]. J Hazard Mater, 2021. DOI:10.1016/j.jhazmat.2020.124491 |
| [3] |
WANG H, DU H, YANG J, et al. PFOS, PFOA, estrogen homeostasis, and birth size in Chinese infants[J]. Chemosphere, 2019, 221: 349-355. DOI:10.1016/j.chemosphere.2019.01.061 |
| [4] |
CAO W, LIU X, LIU X, et al. Perfluoroalkyl substances in umbilical cord serum and gestational and postnatal growth in a Chinese birth cohort[J]. Environment International, 2018, 116: 197-205. DOI:10.1016/j.envint.2018.04.015 |
| [5] |
ALVES R N, MAULVAULT A L, BARBOSA V L, et al. Preliminary assessment on the bioaccessibility of contaminants of emerging concern in raw and cooked seafood[J]. Food and Chemical Toxicology, 2017, 104: 69-78. DOI:10.1016/j.fct.2017.01.029 |
| [6] |
JEONG Y J, BANG S, KIM J, et al. Comparing levels of perfluorinated compounds in processed marine products[J]. Food and Chemical Toxicology, 2019, 126: 199-210. DOI:10.1016/j.fct.2019.01.028 |
| [7] |
QI X, ZHOU J, WANG M, et al. Perfluorinated compounds in poultry products from the Yangtze River Delta and Pearl River Delta regions in China[J]. Science of The Total Environment, 2019, 689: 1079-1086. DOI:10.1016/j.scitotenv.2019.06.258 |
| [8] |
ZHANG M, LI J, ZHANG C, et al. In-situ synthesis of fluorinated magnetic covalent organic frameworks for fluorinated magnetic solid-phase extraction of ultratrace perfluorinated compounds from milk[J]. Journal of Chromatography A, 2020. DOI:10.1016/j.chroma.2019.460773 |
| [9] |
KARRMAN A, DOMINGO J L, LLEBARIA X, et al. Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples[J]. Environmental Science and Pollution Research, 2010, 17(3): 750-758. DOI:10.1007/s11356-009-0178-5 |
| [10] |
LI P, OYANG X, ZHAO Y, et al. Occurrence of perfluorinated compounds in agricultural environment, vegetables, and fruits in regions influenced by a fluorine-chemical industrial park in China[J]. Chemosphere, 2019, 225: 659-667. DOI:10.1016/j.chemosphere.2019.03.045 |
| [11] |
LIANG M, XIAN Y, WANG B, et al. High throughput analysis of 21 perfluorinated compounds in drinking water, tap water, river water and plant effluent from southern China by supramolecular solvents-based microextraction coupled with HPLC-Orbitrap HRMS[J]. Environmental Pollution, 2020. DOI:10.1016/j.envpol.2020.114389 |
| [12] |
XIE H, WEI Y, LI J, et al. In-situ exfoliation of graphitic carbon nitride with metal-organic framework via a sonication-assisted approach for dispersive solid-phase extraction of perfluorinated compounds in drinking water samples[J]. Journal of Chromatography A, 2020. DOI:10.1016/j.chroma.2020.461337 |
| [13] |
TSUDA T, INOUE A, IGAWA T, et al. Seasonal changes of PFOS and PFOA concentrations in Lake Biwa water[J]. Bulletin of Environmental Contamination and Toxicology, 2010, 85(6): 593-597. DOI:10.1007/s00128-010-0116-8 |
| [14] |
WU J, JUNAID M, WANG Z, et al. Spatiotemporal distribution, sources and ecological risks of perfluorinated compounds (PFCs) in the Guanlan River from the rapidly urbanizing areas of Shenzhen, China[J]. Chemosphere, 2020. DOI:10.1016/j.chemosphere.2019.125637 |
| [15] |
郑宇, 路国慧, 邵鹏威, 等. 青藏高原东部过渡区水环境中全氟化合物的分布特征[J]. 环境化学, 2020, 39(5): 1192-1201. ZHENG Y L, GUO H, SHAO P W, et al. Distribution characteristics of perfluorinated compounds in the water environment of the eastern transition zone of the Qinghai-Tibet Plateau[J]. Environmental Chemistry, 2020, 39(5): 1192-1201. |
| [16] |
孙建树, 王世亮. 山东省典型湿地水体和沉积物中全氟辛烷羧酸(PFOA)和全氟辛烷磺酸(PFOS)的污染特征[J]. 环境化学, 2019, 38(07): 1528-1538. SUN J S, WANG S L. Pollution characteristics of perfluorooctane carboxylic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in water bodies and sediments of typical wetlands in Shandong Province[J]. Environmental Chemistry, 2019, 38(07): 1528-1538. |
| [17] |
HE X, LI A, WANG S, et al. Perfluorinated substance assessment in sediments of a large-scale reservoir in Danjiangkou, China[J]. Environmental Monitoring and Assessment, 2018, 190(2): 66. DOI:10.1007/s10661-017-6439-8 |
| [18] |
CAI M, ZHAO Z, YANG H, et al. Spatial distribution of per- and polyfluoroalkyl compounds in coastal waters from the East to South China Sea[J]. Environmental Pollution, 2012, 161: 162-169. DOI:10.1016/j.envpol.2011.09.045 |
| [19] |
SHAFIQUE U, SCHULZE S, SLAWIK C, et al. Gas chromatographic determination of perfluorocarboxylic acids in aqueous samples-A tutorial review[J]. Analytica Chimica Acta, 2017, 949: 8-22. DOI:10.1016/j.aca.2016.10.026 |
| [20] |
WU M, SUN R, WANG M, et al. Analysis of perfluorinated compounds in human serum from the general population in Shanghai by liquid chromatography-tandem mass spectrometry (LC-MS/MS)[J]. Chemosphere, 2017, 168: 100-105. DOI:10.1016/j.chemosphere.2016.09.161 |
| [21] |
ZHU P, LING X, LIU W, et al. Simple and fast determination of perfluorinated compounds in Taihu Lake by SPE-UHPLC-MS/MS[J]. Journal of Chromatography B, 2016, 1031: 61-67. DOI:10.1016/j.jchromb.2016.07.031 |
| [22] |
贺思思, 史亚利, 蔡亚岐, 等. 全氟/多氟化合物分析方法的研究进展[J]. 色谱, 2020, 38(3): 287-296. HE S S, SHI Y L, CAI Y Q, et al. Research progress of analysis methods for perfluorinated/polyfluorinated compounds[J]. Chromatography, 2020, 38(3): 287-296. |
| [23] |
贺锦灿, 张诗韵, 苏榆媛, 等. 典型全氟有机酸类化合物的样品前处理与分析方法研究进展[J]. 色谱, 2020, 38(1): 86-94. HE J C, ZHANG S Y, SU Y Y, et al. Research progress in sample pretreatment and analysis methods of typical perfluorinated organic acids[J]. Chromatography, 2020, 38(1): 86-94. |
| [24] |
吴建刚, 龙强, 肖文, 等. 环境水样中全氟磺酸类和全氟羧酸类化合物分析方法研究进展[J]. 环境化学, 2018, 37(8): 1851-1859. WU J G, LONG Q, XIAO W, et al. Research progress in analytical methods of perfluorosulfonic acid and perfluorocarboxylic acid compounds in environmental water samples[J]. Environmental Chemistry, 2018, 37(8): 1851-1859. |
| [25] |
SPELTINI A, MAIOCCHI M, CUCCA L, et al. Solid-phase extraction of PFOA and PFOS from surface waters on functionalized multiwalled carbon nanotubes followed by UPLC-ESI-MS[J]. Analytical and Bioanalytical Chemistry, 2014, 406(15): 3657-3665. DOI:10.1007/s00216-014-7738-3 |
| [26] |
杨文龙, 郭靖, 杜伟, 等. 超高效液相色谱-新型串联四极杆质谱法测定环境水体与土壤中的全氟辛酸和全氟辛烷磺酸[J]. 环境化学, 2018, 37(12): 2820-2823. YANG W L, GUO J, DU W, et al. Determination of Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid in Environmental Water and Soil by Ultra Performance Liquid Chromatography-New Tandem Quadrupole Mass Spectrometry[J]. Environmental Chemistry, 2018, 37(12): 2820-2823. |
| [27] |
黄东仁, 温裕云, 陈志华, 等. 近岸及河口海水中全氟化合物的固相萃取富集/超高效液相色谱-串联质谱测定[J]. 分析测试学报, 2016, 35(3): 305-310. HUANG D R, WEN Y Y, CHEN Z H, et al. Solid-phase extraction enrichment/ultra high performance liquid chromatography-tandem mass spectrometry determination of perfluorinated compounds in coastal and estuary seawater[J]. Journal of Analysis and Testing, 2016, 35(3): 305-310. DOI:10.3969/j.issn.1004-4957.2016.03.008 |
| [28] |
LACINA O, HRADKOVA P, PULKRABOVA J, et al. Simple, high throughput ultra-high performance liquid chromatography/tandem mass spectrometry trace analysis of perfluorinated alkylated substances in food of animal origin: milk and fish[J]. Journal of Chromatography A, 2011, 1218(28): 4312-4321. DOI:10.1016/j.chroma.2011.04.061 |
| [29] |
CHEN C, LIANG X, WANG J, et al. Development of a polymeric ionic liquid coating for direct-immersion solid-phase microextraction using polyhedral oligomeric silsesquioxane as cross-linker[J]. J Chromatogr A, 2014, 1348: 80-86. DOI:10.1016/j.chroma.2014.04.098 |
| [30] |
APARICIO I, MARTIN J, SANTOS J L, et al. Stir bar sorptive extraction and liquid chromatography-tandem mass spectrometry determination of polar and non-polar emerging and priority pollutants in environmental waters[J]. J Chromatogr A, 2017, 1500: 43-52. DOI:10.1016/j.chroma.2017.04.007 |
| [31] |
董文洪, 杨海, 令狐文生. 串联液质联用仪测定水中全氟辛酸和全氟辛烷磺酸的影响因素分析[J]. 化学世界, 2017, 58(1): 1-6. DONG W H, YANG H, LINGHU W S. Analysis of Influencing Factors in the Determination of Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid in Water by Tandem Liquid Mass Spectrometry[J]. Chemical World, 2017, 58(1): 1-6. |
| [32] |
王懿, 孔德洋, 单正军. 超高效液相色谱串联质谱法对水体中全氟化合物的测定[J]. 安全与环境学报, 2011(6): 88-92. WANG Y, KONG D X, CHAN Z J. Determination of Perfluorinated Compounds in Water by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry[J]. Journal of Safety and Environment, 2011(6): 88-92. DOI:10.3969/j.issn.1009-6094.2011.06.023 |
| [33] |
全国认证认可标准化技术委员会实验室认可分技术委员会. GB/T 27417-2017合格评定化学分析方法确认和验证指南[S]. 北京: 中国标准出版社, 2017. National Certification and Accreditation Standardization Technical Committee Laboratory Accreditation Subcommittee. GB/T 27417-2017 Conformity assessment-Guidance on validation and verification of chemical analytical methods[S]. Beijing: China Standard Press. 2017. |
| [34] |
WANG X F, WANG Q, LI Z G, et al. Determination of 23 perfluorinated alkylated substances in water and suspended particles by ultra-performance liquid chromatography/tandem mass spectrometry[J]. Journal of Environmental Science and Health Part A, 2018, 53(14): 1277-1283. DOI:10.1080/10934529.2018.1528042 |
| [35] |
PAPADOPOULOU A, ROMAN I P, CANALS A, et al. Fast screening of perfluorooctane sulfonate in water using vortex-assisted liquid-liquid microextraction coupled to liquid chromatography-mass spectrometry[J]. Anal Chim Acta, 2011, 691(1/2): 56-61. |
| [36] |
WILLE K, VANDEN B J, NOPPE H, et al. A validated analytical method for the determination of perfluorinated compounds in surface-sea- and sewagewater using liquid chromatography coupled to time-of-flight mass spectrometry[J]. Journal of Chromatography A, 2010, 1217(43): 6616-6622. DOI:10.1016/j.chroma.2010.03.054 |
2. Research and Engineering Center on Aquatic Environment Ecosystem, Shanghai 201306, China;
3. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China
 2022,
Vol. 31
2022,
Vol. 31


