2. 上海海洋大学 水产种质资源发掘与利用教育部重点实验室, 上海 201306;
3. 烟台市海洋经济研究院, 山东 烟台 264003;
4. 长崎大学环中国东海环境资源研究所, 日本 长崎 851-2213
石斑鱼(Epinephelus sp.)隶属于鲈形目(Perciformes)
迄今为止,国内外大多数研究报道中有关鱼类脑垂体分泌的FSHβ和LHβ激素的检测方法主要有RNA水平的检测和蛋白水平的检测。其中:RNA水平的检测主要有RT-PCR、qPCR和原位杂交(in situ hybridization,ISH)技术;蛋白水平的检测主要有蛋白免疫印迹(Western blot)和免疫组织化学(immunohistochemistry,IHC)技术。目前,运用qPCR检测石斑鱼脑垂体中FSHβ和LHβ的技术得到快速发展。例如,在点带石斑鱼(E. malabaricus)[7]、蜂巢石斑鱼(E. merra)[8]、斜带石斑鱼(E. coioides)[9]、褐石斑鱼[10]、乌鳍石斑鱼(E. marginatus)[11]和赤点石斑鱼[12]中,均有FSHβ和LHβ基因含量被检测的报道。然而,运用IHC技术对FSHβ和LHβ蛋白表达定位的检测仅在点带石斑鱼[7]和蜂巢石斑鱼[8]中有少量报道。此外,在一些刚孵化的幼鱼比如点带石斑鱼[7]、蜂巢石斑鱼[8]和赤点石斑鱼[12]中,由于脑垂体器官太小,FSHβ和LHβ的基因含量太低而无法用qPCR技术检测到,所以运用IHC技术对石斑鱼脑垂体中FSHβ和LHβ蛋白表达进行识别和定位,这对于石斑鱼繁殖信息的补充和完善具有重要意义。
本实验以在日本长崎市沿岸海域和冲绳
2018年6—9月,采用渔船捕捞作业和垂钓法相结合的方式,在日本长崎市沿岸海域随机捕获黑边石斑鱼、赤点石斑鱼和纹波石斑鱼,挑选雌性成鱼用于实验,数量分别为6、4和6尾。2019年6—9月,采用渔船捕捞作业和垂钓法相结合的方式,在日本冲绳县濑底岛附近海域随机捕获宝石石斑鱼、尾纹九棘鲈和蜂巢石斑鱼,挑选雌性成鱼用于实验,数量分别为6、7和5尾。
1.2 方法 1.2.1 性腺和脑垂体样品的采集所捕获的石斑鱼成鱼用2-苯氧乙醇麻醉后测量其体长(total length,TL)和体质量(body weight,BW),然后解剖采样。取出性腺,测定质量(gonadal weight,GW)后将其固定在波恩试剂中,参照文献[13]中的方法计算性腺指数(Gonadosomatic index,GSI)。之后打开头盖骨,去除外骨骼后将脑和脑垂体整体固定在波恩试剂中;如无法单独取下脑和脑垂体整体,则将脑和脑垂体连同部分头盖骨一同固定在波恩试剂中。固定在波恩试剂中的样品,24~48 h后转移至70%的乙醇中保存用于组织学分析。
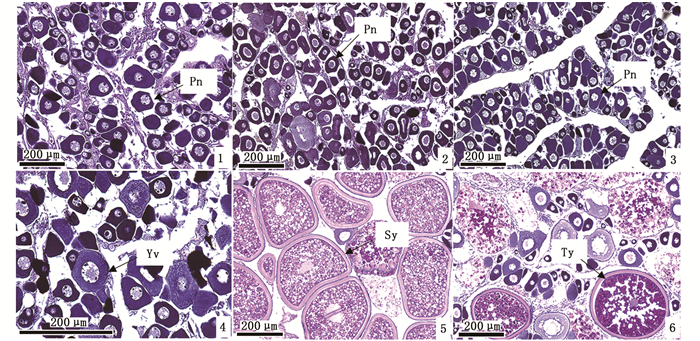
1.2.2 性腺切片的制备和组织学观察对各石斑鱼的性腺进行常规梯度乙醇脱水、二甲苯透明、石蜡包埋和连续切片,厚度为5~7 μm。采用苏木精-伊红法(hematoxylin-eosin,H.E.)对切片进行染色和中性树胶封片,在Olympus FX380型光学显微镜下观察并拍照,以切片图中生殖细胞最发达阶段为性腺的发育阶段。参考文献[14-16]的标准对卵母细胞的发育依次分为核仁外周期(peri-nucleolus stage,Pn)、卵黄囊泡期(yolk vesicle stage,Yv)、次级卵黄球期(secondary yolk stage,Sy)和三级卵黄球期(tertiary yolk stage,Ty)。其中, Pn和Yv阶段为性腺发育未成熟期,Sy和Ty为成熟期。
1.2.3 脑垂体切片的制备宝石石斑鱼、黑边石斑鱼、赤点石斑鱼、尾纹九棘鲈、蜂巢石斑鱼和纹波石斑鱼固定在波恩试剂中脑垂体样品数量分别为6、6、4、7、5和6尾。取固定在波恩试剂中的样本,对脑垂体头盖骨用EDTA脱钙液进行脱钙,然后对脱钙的样本进行常规梯度乙醇脱水(乙醇浓度依次为90%,95%,100%)、乙醇丁醇混合系列(乙醇与丁醇体积比依次为4∶1、3∶2、2∶3、1∶4,最终100%丁醇)、石蜡包埋和连续切片,厚度为5~8 μm。切片用于免疫组织化学(IHC)实验。
1.2.4 免疫组织化学样品固定在波恩试剂后,其脑垂体中的FSHβ和LHβ蛋白抗原会被封闭,因此必须进行抗原暴露实验。将上述切片用梯度乙醇脱水后浸泡在10 mmol/L柠檬酸溶液中,用微波炉加热至90 ℃并恒温保持15 min。随后将切片置于室温下冷却1 h并用蒸馏水洗净。
IHC实验采用抗生物素蛋白-生物素过氧化物酶法,参照文献[7-8]的方法并加以改进。切片浸泡在0.3% H2O2的甲醇中1 h,用PBS缓冲液冲洗干净后在10%山羊血清中封闭15 min,分别滴加由SHIMIZU等[17]赠送的兔抗底鳉(Fundulus heteroclitus)FSHβ(体积比为1∶1 000)和LHβ(体积比为1∶5 000)第一抗体[7-8]在4 ℃下孵育1晚,然后用PBS缓冲液冲洗15 min。用SAB-PO(R)试剂盒(Nichirei Biosciences Inc., Japan)中的山羊抗兔IgG第二抗体溶液孵育1 h,在PBS中漂洗15 min后用链霉亲和素-HRP(horseradish peroxidase)溶液孵育30 min。随后再次在PBS缓冲液中冲洗10 min,最后用试剂盒中的DAB辣根过氧化物酶显色液(DAB horseradish peroxidase)观察免疫反应的信号。切片用苏木精轻轻复染后显微镜观察并摄影,根据FSHβ和LHβ细胞免疫染色的深浅程度判断其信号的强弱。
1.3 数据处理试验数据以平均值±标准差(Mean±SD)表示。采用SPSS 22.0对数据进行比较分析,利用ANOVA检验及Tukey's法进行差异性比较分析,显著性水平设为0.05。
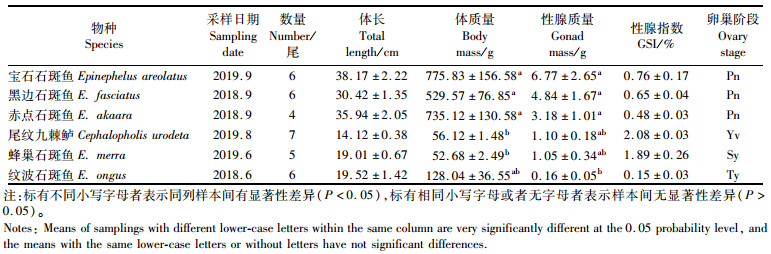
2 结果与分析 2.1 6种石斑鱼采样日期、数量、生长指数及其性腺的发育宝石石斑鱼、黑边石斑鱼和赤点石斑鱼数量分别为6、6和4尾(表 1),其体长均在30 cm以上,体质量在500 g以上。宝石石斑鱼采样日期为2019年9月,卵巢发育为Pn阶段(图版Ⅰ-1);黑边石斑鱼采样日期为2018年9月,卵巢发育为Pn阶段(图版Ⅰ-2);赤点石斑鱼采样日期亦为2018年9月,卵巢发育为Pn阶段(图版Ⅰ-3)。尾纹九棘鲈、蜂巢石斑鱼和纹波石斑鱼数量分别为7、5和6尾,体长14~19 cm,体质量50~130 g。尾纹九棘鲈采样日期为2019年8月,卵巢发育为Yv阶段(图版Ⅰ-4);蜂巢石斑鱼采样日期为2019年6月,卵巢发育为Sy阶段(图版Ⅰ-5);纹波石斑鱼采样日期亦为2018年6月,卵巢发育为Ty阶段(图版Ⅰ-6)。宝石石斑鱼、黑边石斑鱼和赤点石斑鱼的体质量显著高于尾纹九棘鲈和蜂巢石斑鱼(P<0.05),性腺质量显著高于纹波石斑鱼(P<0.05)。宝石石斑鱼、黑边石斑鱼、赤点石斑鱼和尾纹九棘鲈性腺发育处于未成熟阶段;蜂巢石斑鱼和纹波石斑鱼性腺发育处于成熟阶段。
|
表 1 6种石斑鱼的采样时间、数量、生长指数以及性腺发育阶段 Tab.1 Changes of sampling time, number, growth index, and gonadal development in six species of groupers |
|
1.宝石石斑鱼Epinephelus areolatus;2.黑边石斑鱼E. fasciatus;3.赤点石斑鱼E. akaara;4.尾纹九棘鲈Cephalopholis urodeta;5.蜂巢石斑鱼E. merra;6.纹波石斑鱼E. ongus;Pn.核仁外周期;Yv.卵黄囊泡期;Sy.次级卵黄球期;Ty.三级卵黄球期。 1. Areolate grouper Epinephelus areolatus; 2.Blacktip grouper E. fasciatus; 3. Red spotted grouper E. akaara; 4. Flagtail grouper Cephalopholis urodeta; 5. Honeycomb grouper E. merra; 6. White-streaked grouper E. ongus; Pn. Perinucleolus; Yv. Yolk vesicle; Sy. Secondary yolk; Ty. Tertiary yolk. 图版 Ⅰ 6种石斑鱼卵巢发育阶段的切片图 Plate Ⅰ Histological sections of ovary development stages in six species of groupers |
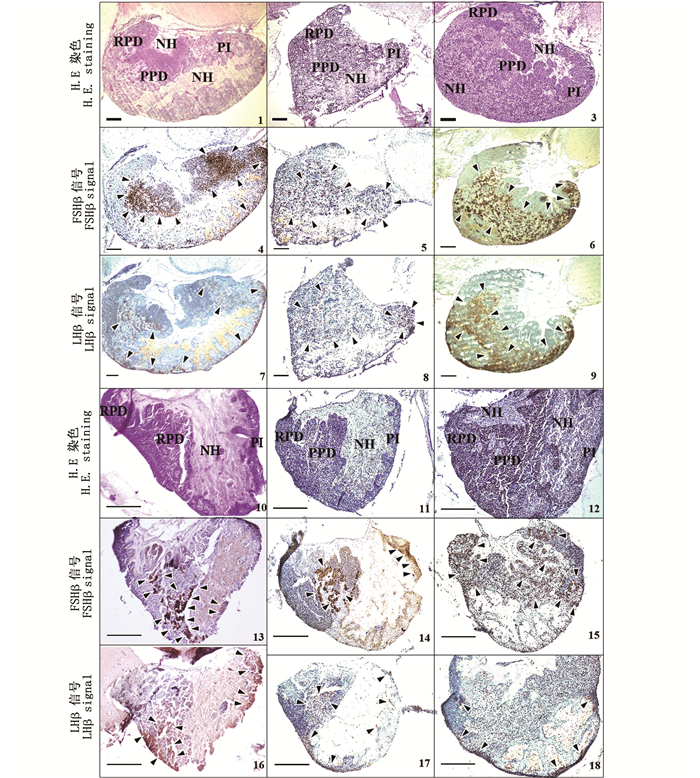
石斑鱼的脑垂体位于间脑腹面,由1个短柄与下丘脑相连,悬垂而下,嵌藏在脑颅副蝶骨背面的小凹窝内[18]。6种石斑鱼的脑垂体呈漏斗状结构,由神经垂体(neurohypophysis,NH)和腺垂体(adenohypophysis,AH)两部分构成,见图版Ⅱ。根据不同的着色区域和边界,腺垂体进一步分为3小部分,从左至右依次为前外侧部(rostral pars distalis,RPD)、中外侧部(proximal pars distalis,PPD)和中间部(pars intermedia,PI,见图版Ⅱ-1,2,3,10,11和12)。
|
1,4和7.宝石石斑鱼Epinephelus areolatus;2,5和8.黑边石斑鱼E. fasciatus;3,6和9.赤点石斑鱼E. akaara;10,13和16.尾纹九棘鲈Cephalopholis urodeta;11,14和17.蜂巢石斑鱼E. merra;12,15和18.纹波石斑鱼E. ongus;NH.神经垂体;RPD.前外侧部;PPD.中外侧部;PI.中间部。箭头表示FSHβ和LHβ阳性信号。比例尺=200 μm。 1, 4 and 7.Areolate grouper Epinephelus areolatus; 2, 5 and 8.Blacktip grouper E. fasciatus; 3, 6 and 9.Red spotted grouper E. akaara; 10, 13 and 16.Flagtail grouper Cephalopholis urodeta; 11, 14 and 17. Honeycomb grouper E. merra; 12, 15 and 18.White-streaked grouper E. ongus. NH. Neurohypophysis; RPD.Rostral pars distalis; PPD. Proximal pars distalis; PI.Pars intermedia. Arrows indicated positive signals. Scale bar=200 μm. 图版 Ⅱ 6种石斑鱼脑垂体结构及垂体中FSHβ和LHβ细胞的切片图 Plate Ⅱ Histological sections of the pituitary structures and immunoreactive signals of FSHβ and LHβ producing cells in six species of adult groupers |
参照文献[7-8]报道IHC实验中对FSHβ和LHβ阳性信号检测的判断,6种石斑鱼脑垂体中FSHβ和LHβ细胞的分布如图版Ⅱ中箭头所示。在宝石石斑鱼中,FSHβ免疫信号主要分布在PPD和PI区域,LHβ信号少量分布在PPD和PI区域(图版Ⅱ-4和7)。在黑边石斑鱼中,FSHβ和LHβ信号少量分布在PPD和PI区域(图版Ⅱ-5和8)。在赤点石斑鱼中,FSHβ和LHβ信号主要分布在PPD和PI区域(图版Ⅱ-6和9)。在尾纹九棘鲈中,FSHβ信号主要分布在PPD区域,少量分布在PI区域,LHβ信号少量分布在PPD和PI区域(图版Ⅱ-13和16)。在蜂巢石斑鱼中,FSHβ信号主要分布在PPD区域,少量分布在PI区域,LHβ信号少量分布在PPD和PI区域(图版Ⅱ-14和17)。在纹波石斑鱼中,FSHβ信号主要分布在PPD和PI区域,LHβ信号少量分布在PPD和PI区域(图版Ⅱ-15和18)。关于免疫信号强度的比较,6种石斑鱼脑垂体中的FSHβ信号均较LHβ强。
宝石石斑鱼、赤点石斑鱼和纹波石斑鱼脑垂体中的NH结构被PPD隔开分为上下两部分(图版Ⅱ-1,3和12,图 1a)。黑边石斑鱼、尾纹九棘鲈和蜂巢石斑鱼脑垂体中的NH结构为一个整体(图版Ⅱ-2,10和11,图 1b)。
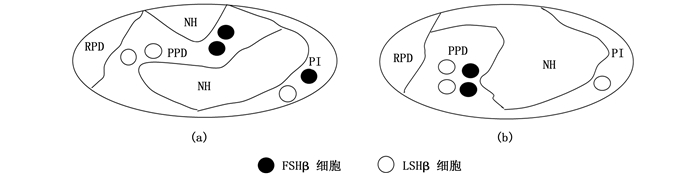
|
宝石石斑鱼Epinephelus areolatus、赤点石斑鱼E. akaara和纹波石斑鱼E. ongus脑垂体结构属于模式图a类型;黑边石斑鱼E. fasciatus、尾纹九棘鲈Cephalopholis urodeta和蜂巢石斑鱼E. merra脑垂体结构属于模式图b类型。 The pattern diagram of pituitary structures in areolate grouper Epinephelus areolatus, red spotted grouper E. akaara and white-streaked grouper E. ongus belongs to type a, and the blacktip grouper E. fasciatus, flagtail grouper Cephalopholis urodeta and honeycomb grouper E. merra belong to type b. 图 1 6种石斑鱼脑垂体结构及垂体中FSHβ和LHβ细胞分布模式图 Fig. 1 Pattern diagram of pituitary structures and immunoreactive signals of FSHβ and LHβ producing cells in six species of adult groupers |
目前,运用IHC技术识别鱼类脑垂体FSHβ和LHβ细胞的研究中,关键因素在于第一抗体能否与抗原相结合,因为抗体与抗原之间存在物种特异性反应。以往的研究结果表明,由日本学者SHIMIZU等[19]开发的兔抗底鳉FSHβ和LHβ抗体不仅被运用在检测底鳉垂体中的FSHβ和LHβ细胞,也被运用于真鲷(Pagrus major)[20]、小口黑鲈(Micropterus dolomieu)[21]和慈鲷(Cichlasoma dimerus)[22]上,表明兔抗底鳉FSHβ和LHβ抗体能够很好地与多种鱼类的抗原相结合,尤其此抗体也适用于石斑鱼比如点带石斑鱼[7]和蜂巢石斑鱼[8]中。因此本研究将其用于包括蜂巢石斑鱼在内的6种石斑鱼中,且检测到阳性信号,表明兔抗底鳉FSHβ或LHβ抗体适用于本实验中的6种石斑鱼。
3.2 6种石斑鱼脑垂体结构的差异以及垂体中FSHβ和LHβ细胞的分布本实验中,6种石斑鱼的脑垂体结构类似,分为NH、RPD、PPD和PI部分,虽与以往的研究结果[23]大致相同,但也有细微差异。在宝石石斑鱼、赤点石斑鱼和纹波石斑鱼中,NH结构被PPD隔开分为上下两部分;而在黑边石斑鱼、尾纹九棘鲈和蜂巢石斑鱼中,NH为一个整体。已有研究表明,在点带石斑鱼[7]、蜂巢石斑鱼[8]、银汉鱼(Odontesthes bonariensis)[24]、底鳉[25]、斑锯脂鲤(Serrasalmus maculatus)[26]、蓝鳍金枪鱼(Thunnus thynnus)[27]和金头鲷(Sparus aurata)[28]的脑垂体结构图中,NH为一个整体,只有在尼罗罗非鱼(Oreochromis niloticus)[29]中,NH被PPD隔开分为上下两部分,表明鱼类脑垂体结构中的NH部分可能由于切片实验时所取部位的不同而有所差异。虽然本实验中的宝石石斑鱼、赤点石斑鱼和纹波石斑鱼的NH结构被PPD隔开分为上下两部分,但在大多数鱼类的结构模式图中,NH结构作为一个整体存在。
6种石斑鱼中,FSHβ细胞均分布在PPD和PI区域。已有研究[8]表明,在蜂巢石斑鱼中,FSHβ细胞主要分布在PPD和PI区域,与本研究结果类似。然而在点带石斑鱼中,FSHβ仅分布在PPD区域[7],表明FSHβ的分布因石斑鱼种类的不同而有所差异。在其他海水鱼类如金头鲷[28]中,FSHβ细胞分布在PPD和PI区域;但在蓝鳍金枪鱼[27]中,FSHβ细胞仅分布在PPD区域,表明在多数海水鱼类中,FSHβ的分布存在两种模式,分布于PPD和PI区域(图 1a)或仅分布于PPD区域(图 1b)。6种石斑鱼FSHβ细胞的分布见图 1a。此外,本实验的6种石斑鱼中,LHβ细胞均分布在PPD和PI区域。在点带石斑鱼[7]、蜂巢石斑鱼[8]、蓝鳍金枪鱼[27]、金头鲷[28]和地中海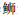
已有研究表明:宝石石斑鱼[31-32]在1—5月,体长超过28 cm时出现成熟雌性个体;黑边石斑鱼[33]在7—8月,体长超过23.5 cm时出现成熟雌性个体;赤点石斑鱼[34]在5—6月,体长超过20 cm时出现成熟雌性个体;尾纹九棘鲈[35]成熟雌性个体采集时间在5月,体长在14.9~15.7 cm。本实验中宝石石斑鱼、黑边石斑鱼和赤点石斑鱼体长分别为39.4 cm、32.0 cm和43.4 cm,采样日期在9月初;尾纹九棘鲈体长为14.8 cm,采样日期在8月底。尽管上述各石斑鱼的体长均已接近或超过已有报道中成熟时的体长规格,但性腺发育仍为Pn或Yv未成熟卵母细胞阶段,可能原因是实验鱼的采样日期均在其繁殖季节之后。已有研究[36-38]表明,蜂巢石斑鱼的繁殖季节开始于5月,在6月达到峰值,成熟体长为18.1 cm左右。纹波石斑鱼的繁殖季节推断在4—7月,成熟体长为18.9 cm左右[39]。本实验中蜂巢石斑鱼和纹波石斑鱼由于其采集日期正好在6月的繁殖季节,且体长也达到了成熟规格,因此性腺发育分别为Sy和Ty成熟阶段。
本实验中6种石斑鱼的性腺发育为未成熟和成熟阶段,且各发育阶段FSHβ信号均较LHβ强,推断在石斑鱼性腺发育过程中FSHβ较LHβ重要。然而,由于FSHβ和LHβ在鱼种中具有物种特异性,因此仅通过免疫信号的强弱无法准确判断FSHβ和LHβ在石斑鱼性腺发育过程中的角色。此外,尽管宝石石斑鱼、黑边石斑鱼、赤点石斑鱼和尾纹九棘鲈体长均已接近或超过成熟时的体长规格,但性腺发育仍为未成熟阶段,而蜂巢石斑鱼和纹波石斑鱼为成熟阶段。基于本实验中各石斑鱼性腺发育程度的差异性,因此无法准确判断FSHβ和LHβ在石斑鱼成熟和繁殖过程中的角色。已有研究[8]表明:在成年雌性蜂巢石斑鱼中,LHβ的基因表达和免疫信号均较FSHβ强;此外,口服FSHβ激素能诱导蜂巢石斑鱼由雌性逆转为雄性,表明FSHβ和LHβ与雌雄性蜂巢石斑鱼的性成熟密切相关。类似的结果也出现在真鲷中,FSHβ可能对其雄性配子的发育起重要作用,而对雌性配子无影响[20, 40]。然而在点带石斑鱼中,FSHβ和LHβ免疫信号出现在初级卵母细胞阶段,其角色可能与性分化无关[7]。此外,在鲑科(Oncorhynchus)[41-44]、星康吉鳗(Conger myriaster)[45]、日本鳗鲡(Anguilla japonica)[46]和欧洲鲈(Dicentrarchus labrax)[47]中,FSHβ对性腺早期发育如精子和卵子的生长具有重要影响,而LHβ主要调控性腺发育的成熟阶段如排卵和排精等行为。由于FSHβ和LHβ在鱼种中具有多样性和复杂性,因此仅凭免疫信号的强弱无法探讨FSHβ和LHβ在石斑鱼发育过程中的角色。进一步的研究还需要结合运用荧光定量分子生物学实验综合讨论FSHβ和LHβ在石斑鱼性腺发育过程中的角色。
| [1] |
LIAO I C, LEAÑO E M. The aquaculture of groupers[M]. Taiwan, China: Asian Fisheries Society, 2008: 1-242.
|
| [2] |
农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 中国渔业统计年鉴-2020[M]. 北京: 中国农业出版社, 2020: 22-23. Fishery Administration Bureau of Ministry of Agriculture and Rural Areas, National Aquatic Technology Extension Station, The China Society of Fisheries. China fishery statistical yearbook[M]. Beijing: China Agriculture Press, 2020: 22-23. |
| [3] |
NAMBU T. Ⅱ-7. Seed production and aquaculture technology of red spotted grouper Epinephelus akaara[J]. Nippon Suisan Gakkaishi, 2004, 80(6): 1000. |
| [4] |
李广丽, 刘晓春, 林浩然. 芳香化酶抑制剂letrozole对赤点石斑鱼(Epinephelus akaara)性逆转的作用[J]. 生理学报, 2005, 57(4): 473-479. LI G L, LIU X C, LIN H R. Aromatase inhibitor letrozole induces sex inversion in the protogynous red spotted grouper (Epinephelus akaara)[J]. Acta Physiologica Sinica, 2005, 57(4): 473-479. DOI:10.3321/j.issn:0371-0874.2005.04.010 |
| [5] |
KAWABE K, KOHNO H. Morphological development of larval and juvenile blacktip grouper, Epinephelus fasciatus[J]. Fisheries Science, 2009, 75(5): 1239-1251. DOI:10.1007/s12562-009-0128-7 |
| [6] |
CHUDA H. Ⅱ-6. Seed production and aquaculture technology of longtooth grouper Epinephelus bruneus[J]. Nippon Suisan Gakkaishi, 2014, 80(6): 999. DOI:10.2331/suisan.80.999 |
| [7] |
MURATA R, KOBAYASHI Y, KARIMATA H, et al. The role of pituitary gonadotropins in gonadal sex differentiation in the protogynous Malabar grouper, Epinephelus malabaricus[J]. General and Comparative Endocrinology, 2012, 178(3): 587-592. DOI:10.1016/j.ygcen.2012.07.012 |
| [8] |
KOBAYASHI Y, ALAM M A, HORIGUCHI R. Sexually dimorphic expression of gonadotropin subunits in the pituitary of protogynous honeycomb grouper (Epinephelus merra): Evidence that follicle-stimulating hormone (FSH) induces gonadal sex change[J]. Biology of Reproduction, 2010, 82(6): 1030-1036. DOI:10.1095/biolreprod.109.080986 |
| [9] |
ZHANG W M, ZHANG Y, ZHANG L H, et al. The mRNA expression of P450 aromatase, gonadotropin β-subunits and FTZ-F1 in the orange-spotted grouper (Epinephelus coioides) during 17α-methyltestosterone-induced precocious sex change[J]. Molecular Reproduction and Development, 2007, 74(6): 665-673. DOI:10.1002/mrd.20642 |
| [10] |
HUR S P, LIM B S, HWANG I J, et al. Masculinization in juvenile longtooth grouper, Epinephelus bruneus, with aromatase inhibitor: changes in GtH subunit mRNA expression and steroids hormone levels[J]. Animal Cells and Systems, 2012, 16(2): 127-134. DOI:10.1080/19768354.2011.607515 |
| [11] |
DE O. GARCIA C E, ARA?JO B C, MELLO P H, et al. Involvement of pituitary gonadotropins, gonadal steroids and breeding season in sex change of protogynous dusky grouper, Epinephelus marginatus (Teleostei: Serranidae), induced by a non-steroidal aromatase inhibitor[J]. General and Comparative Endocrinology, 2013, 192: 170-180. DOI:10.1016/j.ygcen.2013.06.012 |
| [12] |
KIM H K, KIM J H, BAEK H J, et al. Gene Expression of aromatases, steroid receptor, GnRH and GTHs in the brain during the formation of ovarian cavity in red spotted grouper, Epinephelus akaara[J]. Development & Reproduction, 2016, 20(4): 367-377. |
| [13] |
SONG J, NAGAE M, SOYANO K. Changes in plasma vitellogenin and estradiol-17β levels during the gonadal development of the female Japanese common goby Acanthogobius flavimanus[J]. Aquaculture Science, 2017, 65(4): 303-310. |
| [14] |
YAMAMOTO K, YAMAZAKI F. Rhythm of development in the oocyte of the gold-fish, Carassius auratus[J]. Bulletin of the Faculty of Fisheries Hokkaido University, 1961, 12(2): 93-110. |
| [15] |
崔丹, 刘志伟, 刘南希, 等. 金钱鱼性腺发育及其组织结构观察[J]. 水产学报, 2013, 37(5): 696-704. CUI D, LIU Z W, LIU N X, et al. Histological study on the gonadal development of Scatophagus argus[J]. Journal of Fisheries of China, 2013, 37(5): 696-704. |
| [16] |
王盈颖. 赤点石斑鱼和青石斑鱼性分化过程中性腺发育的研究[D]. 厦门: 厦门大学, 2015. WANG Y Y. Gonad development during sexual differentiation in Epinephelus akaara and E. awoara[D]. Xiamen: Xiamen University, 2015. |
| [17] |
SHIMIZU A, YAMASHITA M. Purification of mummichog (Fundulus heteroclitus) gonadotropins and their subunits, using an immunochemical assay with antisera raised against synthetic peptides[J]. General and Comparative Endocrinology, 2002, 125(1): 79-91. DOI:10.1006/gcen.2001.7741 |
| [18] |
魏华, 吴垠. 鱼类生理学[M]. 2版. 北京: 中国农业出版社, 2011: 263-264. WEI H, WU Y. Fish physiology[M]. 2nd ed. Beijing: China Agricultural Press, 2011: 263-264. |
| [19] |
SHIMIZU A, TANAKA H, KAGAWA H. Immunocytochemical applications of specific antisera raised against synthetic fragment peptides of mummichog GtH subunits: examining seasonal variations of gonadotrophs (FSH cells and LH cells) in the mummichog and applications to other acanthopterygian fishes[J]. General and Comparative Endocrinology, 2003, 132(1): 35-45. DOI:10.1016/S0016-6480(03)00037-6 |
| [20] |
GEN K, OKUZAWA K, SENTHILKUMARAN B, et al. Unique expression of gonadotropin-I and-Ⅱ subunit genes in male and female red seabream (Pagrus major) during sexual maturation[J]. Biology of Reproduction, 2000, 63(1): 308-319. DOI:10.1095/biolreprod63.1.308 |
| [21] |
SHIMIZU A, TANAKA H, KAGAWA H. Immunocytochemical applications of specific antisera raised against synthetic fragment peptides of mummichog GtH subunits: examining seasonal variations of gonadotrophs (FSH cells and LH cells) in the mummichog and applications to other acanthopterygian fishes[J]. General and Comparative Endocrinology, 2003, 132(1): 35-45. DOI:10.1016/S0016-6480(03)00037-6 |
| [22] |
PANDOLFI M, LO NOSTRO F L, SHIMIZU A, et al. Identification of immunoreactive FSH and LH cells in the cichlid fish Cichlasoma dimerus during the ontogeny and sexual differentiation[J]. Anatomy and Embryology, 2006, 211(5): 355-365. DOI:10.1007/s00429-006-0086-0 |
| [23] |
舒琥, 刘晓春, 张勇, 等. 赤点石斑鱼脑垂体超微结构的初步研究[J]. 中山大学学报(自然科学版), 2008, 47(4): 68-71. SHU H, LIU X C, ZHANG Y, et al. The preliminary study on the ultrastructure of pituitary of Epinephelus akaara[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2008, 47(4): 68-71. |
| [24] |
MIRANDA L A, STR?SSMANN C A, SOMOZA G M. Immunocytochemical identification of GtH1 and GtH2 cells during the temperature-sensitive period for sex determination in pejerrey, Odontesthes bonariensis[J]. General and Comparative Endocrinology, 2001, 124(1): 45-52. DOI:10.1006/gcen.2001.7687 |
| [25] |
SHIMIZU A, YAMASHITA M. Purification of mummichog (Fundulus heteroclitus) gonadotropins and their subunits, using an immunochemical assay with antisera raised against synthetic peptides[J]. General and Comparative Endocrinology, 2002, 125(1): 79-91. DOI:10.1006/gcen.2001.7741 |
| [26] |
NOBREGA R H, DE JESUS L W O, HONJI R M, et al. Characterization of gonadotropic cells during continuous and seasonal spermatogenesis of two freshwater fish species: a histochemical and immunohistochemical study[J]. Fish Physiology and Biochemistry, 2017, 43(1): 51-63. DOI:10.1007/s10695-016-0267-6 |
| [27] |
KAGAWA H, KAWAZOE I, TANAKA H, et al. Immunocytochemical identification of two distinct gonadotropic cells (GTH I and GTH Ⅱ) in the pituitary of Bluefin tuna, Thunnus thynnus[J]. General and Comparative Endocrinology, 1998, 110(1): 11-18. DOI:10.1006/gcen.1997.7049 |
| [28] |
GARCÍA AYALA A, VILLAPLANA M, GARCÍA HERNÁNDEZ M P, et al. FSH-, LH-, and TSH-expressing cells during development of Sparus aurata L. (Teleostei). An immunocytochemical study[J]. General and Comparative Endocrinology, 2003, 134(1): 72-79. DOI:10.1016/S0016-6480(03)00198-9 |
| [29] |
KASPER R S, SHVED N, TAKAHASHI A, et al. A systematic immunohistochemical survey of the distribution patterns of GH, prolactin, somatolactin, β-TSH, β-FSH, β-LH, ACTH, and α-MSH in the adenohypophysis of Oreochromis niloticus, the Nile tilapia[J]. Cell and Tissue Research, 2006, 325(2): 303-313. DOI:10.1007/s00441-005-0119-7 |
| [30] |
GARCIÍA HERNÁNDEZ M P, GARCIÍA AYALA A, AGULLEIRO B, et al. Development of a homologous radioimmunoassay for Mediterranean yellowtail (Seriola dumerilii, Risso 1810) LH[J]. Aquaculture, 2002, 210(1/4): 203-218. |
| [31] |
ABDUL KADIR N H, MAT PIAH R, AMBAK M A, et al. Reproductive aspects of areolate grouper, Epinephelus areolatus and six-barred grouper, E. sexfasciatus from Terengganu waters, Malaysia[J]. AACL Bioflux, 2016, 9(6): 1372-1379. |
| [32] |
LIN Y J, ROA-URETA R H, RABAOUI L, et al. Association to vegetated habitats and different vulnerability to habitat degradation for two fish species, Epinephelus areolatus (Serranidae) and Siganus canaliculatus (Siganidae), from the western Arabian Gulf[J]. Marine Pollution Bulletin, 2019, 141: 482-492. DOI:10.1016/j.marpolbul.2019.03.011 |
| [33] |
BARICHE M, HEEMSTRA P. First record of the blacktip grouper Epinephelus fasciatus (Teleostei: Serranidae) in the Mediterranean Sea[J]. Marine Biodiversity Records, 2012, 5: e1. DOI:10.1017/S1755267211000509 |
| [34] |
KAYANO Y, ODA T. Growth and spawning of red spotted grouper, Epinephelus akaara, under artificial rearing condition[J]. Aquaculture Science, 1994, 42(3): 419-425. |
| [35] |
NAKAI T, SANO M. Evidence of protogynous hermaphroditism in the darkfin hind Cephalopholis urodeta (Serranidae) at Iriomote Island, southern Japan[J]. Fisheries Science, 2002, 68(3): 697-699. DOI:10.1046/j.1444-2906.2002.00479.x |
| [36] |
LEE Y D, PARK S H, TAKEMURA A, et al. Histological observations of seasonal reproductive and lunar-related spawning cycles in the female honeycomb grouper Epinephelus merra in Okinawan waters[J]. Fisheries Science, 2002, 68(4): 872-877. DOI:10.1046/j.1444-2906.2002.00505.x |
| [37] |
SOYANO K, MASUMOTO T, TANAKA H, et al. Lunar-related spawning in honeycomb grouper, Epinephelus merra[J]. Fish Physiology and Biochemistry, 2003, 28(1-4): 447-448. DOI:10.1023/B:FISH.0000030625.40402.18 |
| [38] |
左永松, 仲地政人, 中村将, 等. HCG与LHRHa对雌性蜂巢石斑鱼产卵期间生理变化的影响及比较[J]. 上海海洋大学学报, 2013, 22(6): 841-848. ZUO Y S, NAKACHI M, NAKAMURA M, et al. Impacts of artificial maturation inducing hormone HCG and LHRHa on the physiological fluctuation of female honeycomb grouper, Epinephelus merra, during its spawning season and comparison between the two hormones[J]. Journal of Shanghai Ocean University, 2013, 22(6): 841-848. |
| [39] |
OHTA I, EBISAWA A. Reproductive biology and spawning aggregation fishing of the white-streaked grouper, Epinephelus ongus, associated with seasonal and lunar cycles[J]. Environmental Biology of Fishes, 2015, 98(6): 1555-1570. DOI:10.1007/s10641-015-0382-8 |
| [40] |
KAGAWA H, TANAKA H, OKUZAWA K, et al. GTH Ⅱ but not GTH Ⅰ induces final maturation and the development of maturational competence of oocytes of red seabream in vitro[J]. General and Comparative Endocrinology, 1998, 112(1): 80-88. DOI:10.1006/gcen.1998.7133 |
| [41] |
TYLER C R, SUMPTER J P, KAWAUCHI H, et al. Involvement of gonadotropin in the uptake of vitellogenin into vitellogenic oocytes of the rainbow trout, Oncorhynchus mykiss[J]. General and Comparative Endocrinology, 1991, 84(2): 291-299. DOI:10.1016/0016-6480(91)90052-8 |
| [42] |
PLANAS J V, SWANSON P, DICKHOFF W W. Regulation of testicular steroid production in vitro by gonadotropins (GTH Ⅰ and GTH Ⅱ) and cyclic AMP in coho salmon (Oncorhynchus kisutch)[J]. General and Comparative Endocrinology, 1993, 91(1): 8-24. DOI:10.1006/gcen.1993.1099 |
| [43] |
PRAT F, SUMPTER J P, TYLER C R. Validation of radioimmunoassays for two salmon gonadotropins (GTH Ⅰ and GTH Ⅱ) and their plasma concentrations throughout the reproductive cycle in male and female rainbow trout (Oncorhynchus mykiss)[J]. Biology of Reproduction, 1996, 54(6): 1375-1382. DOI:10.1095/biolreprod54.6.1375 |
| [44] |
TYLER C R, POTTINGER T G, COWARD K, et al. Salmonid follicle-stimulating hormone (GtH I) mediates vitellogenic development of oocytes in the rainbow trout, Oncorhynchus mykiss[J]. Biology of Reproduction, 1997, 57(5): 1238-1244. DOI:10.1095/biolreprod57.5.1238 |
| [45] |
KAJIMURA S, YOSHIURA Y, SUZUKI M, et al. Changes in the levels of mRNA coding for gonadotropin Ⅰβ and Ⅱβ subunits during vitellogenesis in the common Japanese conger Conger myriaster[J]. Fisheries Science, 2001, 67(6): 1053-1062. DOI:10.1046/j.1444-2906.2001.00361.x |
| [46] |
SUETAKE H, OKUBO K, SATO N, et al. Differential expression of two gonadotropin (GTH) β subunit genes during ovarian maturation induced by repeated injection of salmon GTH in the Japanese eel Anguilla japonica[J]. Fisheries Science, 2002, 68(2): 290-298. DOI:10.1046/j.1444-2906.2002.00424.x |
| [47] |
MOLÉS G, GÓMEZ A, CARRILLO M, et al. Determination of Fsh quantity and bioactivity during sex differentiation and oogenesis in European sea bass[J]. Biology of Reproduction, 2011, 85(4): 848-857. DOI:10.1095/biolreprod.111.091868 |
2. Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China;
3. Yantai Institute of Marine Economy, Yantai 264003, China;
4. Institute for East China Sea Research, Organization for Marine Science and Technology, Nagasaki University, Nagasaki 851-2213, Japan
 2022,
Vol. 31
2022,
Vol. 31


