2. 上海海洋大学水产科学国家级实验教学示范中心, 上海 201306;
3. 上海海洋大学农业农村部淡水水产种质资源重点实验室, 上海 201306;
4. 上海市沁淼生物科技有限公司, 上海 201802
美国鲥鱼(Alosa sapidissima)俗称美国鲱鱼,简称美洲鲥,隶属于鲱形总目(Clupeomorpha)鲱形目(Clupeiformes)鲱科(Clupeidae)鲥属(Alosa)[1], 是一种生殖洄游性鱼类[2]。美国鲥鱼因其肉质鲜美、营养价值极高而被作为特优经济鱼类被各国引进养殖。2003年美国鲥鱼被引入我国,在多省市进行人工养殖试验的尝试,逐渐发展成为国内较名贵的养殖品种[3-4]。但由于美国鲥鱼人工养殖技术有待完善,病害防治技术滞后,养殖成功率仍有待提升。
近几年鱼类结节病是不断暴发的常见鱼类疾病之一。已有乌鳢(Ophiocephalus argus)[5]、花鲈(Lateolabrax japonicus)[6]、大黄鱼(Larimichthys crocea)[7]诺卡氏菌病或结节病以及大西洋鲷(Sparus aurata)的巴氏杆菌病[8]、美人鱼发光杆菌引起的卵形鲳鲹(Trachinotus ovatus)结节病[9]、嗜水气单胞菌引起花鲈细菌性结节病[10]和 鱼诺卡氏菌引起卵形鲳
鱼诺卡氏菌引起卵形鲳 结节病[11]等。除细菌感染致病外,还有文献表明黏孢子虫(Myxosporea) [12-13]、类立克次体(Rickettsia-like organism, RLO)[14-16]等也可导致鱼类内脏结节症。患有该病的鱼类初期症状不易察觉。随着病情的加重,患病鱼体表现出体质消瘦、摄食异常、离群独游等症状,患病后期鱼体逐渐死亡,已基本无法救治,死亡率极高[17-19]。近几年在上海和其他美国鲥鱼养殖场陆续出现以内脏器官分布明显的白色结节为主要临床症状的相关疾病(暂定名为美国鲥鱼内脏结节病), 病因不明,无法得到有效控制。为深入了解该病的病因和致病机理,寻找有效的防治方法开展本实验研究,为美国鲥鱼内脏结节病的防治提供参考。
结节病[11]等。除细菌感染致病外,还有文献表明黏孢子虫(Myxosporea) [12-13]、类立克次体(Rickettsia-like organism, RLO)[14-16]等也可导致鱼类内脏结节症。患有该病的鱼类初期症状不易察觉。随着病情的加重,患病鱼体表现出体质消瘦、摄食异常、离群独游等症状,患病后期鱼体逐渐死亡,已基本无法救治,死亡率极高[17-19]。近几年在上海和其他美国鲥鱼养殖场陆续出现以内脏器官分布明显的白色结节为主要临床症状的相关疾病(暂定名为美国鲥鱼内脏结节病), 病因不明,无法得到有效控制。为深入了解该病的病因和致病机理,寻找有效的防治方法开展本实验研究,为美国鲥鱼内脏结节病的防治提供参考。
采样选自上海嘉定某养殖场临床症状明显的濒死和患病美国鲥鱼,于2017年8月放苗开始养殖,发病期间日均死亡3~5条。于2018年8月初(5尾)、9月初(7尾)、9月底(10尾)采集样品鱼,共计22尾,现场碎冰装箱,送至上海海洋大学水族宠物医学实验室,病鱼体质量为200~350 g, 体长为22~35 cm。对3个批次的病鱼样品进行临床诊断和病理解剖,初步筛选并排除其他疾病病鱼后,确定8尾样品(编号1~8), 进一步病理检验和诊断。
1.2 方法 1.2.1 临床症状和病理解剖观察分别记录病鱼样品1~8号的临床症状并汇总病理解剖后病鱼体表及其各组织器官的病理变化特征,分别拍照、记录。对结果进行初步诊断分析,以确定典型的病变器官和组织。
1.2.2 H.E染色组织病理学制片及观察取患病美国鲥鱼心脏、鳃、肝脏、肾脏、脾脏、肠道和幽门盲囊等组织,用解剖刀切至0.1 cm3的组织小块置于体积分数为10%的中性甲醛中固定24 h, 流水冲洗数小时,经50%~100%各级乙醇逐级脱水、二甲苯透明、石蜡包埋、组织切片(切片厚度5 μm)、摊片、烤片、H.E染色、中性树脂封片,在LeicaDM3000显微镜下观察并采集图片。
1.2.3 马松染色病理制片及观察根据马松染色试剂盒(Solarbio-G1345)所提供的实验步骤,对病鱼样品7号脾脏组织染色制片,在LeicaDM3000显微镜下观察并采集图片。
1.2.4 抗酸染色病理制片及观察对病鱼样品4、6和7号的肝脏、脾脏和肾脏组织块进行石蜡包埋、组织制片,后依照抗酸杆菌染色试剂盒(Ziehl-Neelsen热染法,Solarbio-G1274)说明书分别进行染色、封片,在LeicaDM3000显微镜下观察并采集图片。
1.2.5 细胞病理制片及观察患病美国鲥鱼6号肝、脾、肾取材组织,取1 mm3, 投入2.5%戊二醛电镜专用固定液4 ℃固定2~4 h, 0.1 mol/L磷酸缓冲液PBS(pH=7.4)漂洗3次,每次15 min。后经质量分数为1%的锇酸,室温(20 ℃)固定2 h。后经质量分数为50%→70%→80%→90%→95%→100%→100%的乙醇和质量分数为100%→100%的丙酮梯度脱水,每次15 min; 丙酮渗透过夜;纯812包埋剂包埋入包埋板;60~80 μm超薄切片、铀铅染色,最后在HITACHI-HT7700透射电镜下观察并采集图片。
2 结果 2.1 患病美国鲥鱼临床症状和病理解剖特征患病美国鲥鱼发病初期均无明显临床症状,随着病情的不断发展开始出现摄食量降低、离群独游等现象;患病末期鱼体活力明显降低,受外界刺激不敏感,不日而亡。临床观察结果(图版Ⅰ)显示:病鱼样品1号鱼体表面出现若干轻微擦伤,腹部轻微肿胀、鳞片下出血(图版Ⅰ-1), 2号胸鳍基部出血(图版Ⅰ-2), 其他病鱼样品体表无明显损伤。经解剖发现病鱼样品3号和5号肝脏表面呈深红色(图版Ⅰ-3, 5), 其中3号肝脏破裂出血(图版Ⅰ-3), 6号肾脏组织坏死至糜烂(图版Ⅰ-6)。3、4和7号腹腔内肝脏、肾脏和脾脏(图版Ⅰ-4, 7, 8)等内脏器官表面有多个直径1~2 mm的白色结节,病鱼样品8号内脏腔中含大量腹水,肝脏呈黄色(图版Ⅰ-9)。显微镜检患病鱼体鳃部、体表和内脏组织液均未发现寄生虫寄生。

|
1.病鱼样品1号体表擦伤部位(↖);2.病鱼样品2号胸鳍基部破裂出血;3.病鱼样品3号肝脏充血且表面分布白色结节;4.病鱼样品4号肝脏表面分布大量白色结节(↖);5.病鱼样品5号肝脏淤血,肾脏表面布满白色结节(↖);6.病鱼样品6号肾脏坏死糜烂(↖);7.病鱼样品7号肾脏表面分布结节,内脏腔腹水(↖);8.脾脏表面白色结节(↖);9.病鱼样品8号内脏腔腹水,肝脏呈黄色(↖)。 1. Body surface abrasions in sick American shad No.1 (↖); 2. Pectoral fin rupture and bleeding in sick American shad No.2; 3. Liver is congested with white nodules on the surface in sick American shad No.3; 4. Large number of white nodules on the liver's surface in sick American shad No.4(↖); 5. Liver congestion in sick American shad No.5, the surface of the kidney is covered with white nodules(↖); 6. Renal necrosis in sick American shad No.6(↖); 7. Visceral ascites and nodules on the surface of the kidney in sick American shad No.7(↖); 8. White nodules on spleen surface(↖); 9. Visceral cavity ascites in sick American shad No.8 and liver turns yellow(↖). 图版 Ⅰ 病鱼样品临床解剖观察 Plate Ⅰ Observation of clinical anatomy of sick fish samples |
肝脏、脾脏、肾脏临床解剖所见的白色结节为大量肉芽肿聚集形成。显微镜下观察发现患病美国鲥鱼肝脏、肾脏和脾脏等组织内含有大量的炎性肉芽肿,同时还伴随有其他组织病理变化(图版Ⅱ)。病变较轻的组织肉芽肿数量较少且直径小,较为严重的病变组织间肉芽肿分布数量多且直径较大(图版Ⅱ-5, 6), 病变最严重时组织内部肉芽肿大面积融合演变为大的肉芽肿集合体,几乎充盈在整个组织内部(图版Ⅱ-6), 占据原有的组织细胞位置。

|
1.病鱼样品3号肝组织内典型肉芽肿结构,内(I)、外(O)两层;2.4号肾组织内典型肉芽肿,内(I)、外(O)两层;3.脾脏内典型肉芽肿;4.6号肝组织间肉芽肿形成初期,炎性细胞聚集;5. 4号肾组织内分布大量肉芽肿(▲),肾小管脱落(←);6.4号肝脏内多个肉芽肿外包膜融合(←);7. 8号肝脏内脂肪变性,空泡化严重;8.5号脾脏内分布大量炎性细胞;9.6号肾组织间分布大量炎性细胞(→),肾小管萎缩并脱落(←);10.8号脾组织中脾髓间分布大量炎性细胞和红细胞;11.2号心肌细胞间分布大量炎性细胞;12.4号鳃小片上呼吸上皮细胞全部脱落,结构变形(←);13. 8号肠道黏膜层Mu与固有层TP分离,柱状细胞坏死脱落;14.8号肠道黏膜层Mu分布大量杯状细胞(←);15.8号幽门盲囊黏膜层Mu与固有层TP分离。 1. Typical granuloma structure in liver tissue in sick American shad No.3, inner (I), outer (O) two layers; 2. Typical granuloma in sick American shad No.4 kidney with two layers of internal (I) and external (O); 3. Typical granuloma in the spleen; 4.Early stage of granuloma formation in liver tissue and inflammatory cell aggregation in sick American shad No.6; 5. A large number of granulomas are observed in sick American shad No.4 kidney (▲), and the renal tubules are shed (←); 6.Multiple granulomas in sick American shad No.4 liver are fused with an outer membrane (←); 7. Fatty degeneration in sick American shad No.8 liver with severe vacuolation; 8. Lots of inflammatory cells in sick American shad No.5 spleen; 9. A large number of inflammatory cells in the kidney (→) and the renal tubules atrophy as well as fall off in No.6 sick American shad (←); 10. Numerous inflammatory cells and red blood cells in the spleen and spinal cord of spleen tissue in sick American shad No.8; 11. Excessive inflammatory cells in sick American shad No.2 myocardial cells; 12. Respiratory epithelial cells on gill plate all shed and the structure is deformed in No.4 sick American shad (←); 13. The intestinal mucosal layer Mu was separated from the propria layer (TP) in sick American shad No.8, and the columnar cells were necrotic and shed; 14.Numerous goblet cells distributed in No.8 sick American shad intestinal mucosa (Mu) (←); 15. The mucosa(Mu) of the pyloric caecum was separated from the lamina propria(TP) in sick American shad No.8. 图版 Ⅱ 病鱼样品组织病理学观察 Plate Ⅱ Observation of histopathology of sick fish samples |
肝脏组织细胞内嗜酸性粒细胞和巨噬细胞大量分布在肉芽肿纤维包膜的外缘(图版Ⅱ-1)。肝细胞索结构消失,组织间分布着大量脂肪颗粒,肝脏细胞肿胀变性(图版Ⅱ-7)。
肾脏组织间分布大量血细胞和炎性细胞,肾小管上皮细胞萎缩并脱落(图版Ⅱ-9)。
脾脏组织淋巴细胞数量减少,脾细胞间质中含大量炎性细胞分布(图版Ⅱ-8)。脾髓间含大量红细胞(图版Ⅱ-10)。
心肌组织内除可见少量嗜酸性粒细胞分布外未见较明显病变(图版Ⅱ-11)。
鳃组织的鳃小片呼吸上皮细胞大面积脱落,仅剩下支持细胞(图版Ⅱ-12)。
肠组织黏膜层与固有层分离,大量的柱状细胞脱落坏死(图版Ⅱ-13)。黏膜层杯状细胞异常增多(图版Ⅱ-14)。
幽门盲囊组织黏膜层与固有层完全脱落,严重时黏膜层脱落消失(图版Ⅱ-15)。
2.3 脏器组织内肉芽肿的结构和形成各组织内典型的肉芽肿由纤维细胞包膜、坏死组织与菌体混合物构成。马松染色显示肉芽肿红色外层(图版Ⅲ-2)为纤维组织结构,确定肉芽肿的最外层包膜是由成纤维细胞和纤维细胞组成的层状结构。成熟且典型的肉芽肿组织中心表现为干酪样坏死,中心包含大量坏死的组织细胞、白细胞和巨噬细胞,呈不规则形状分布(图版Ⅱ-3, Ⅲ-1)。在肉芽肿中心分布有大量的细菌并侵入至纤维包膜中。抗酸染色显示该菌呈紫红色,短杆状(图版Ⅳ-5)。
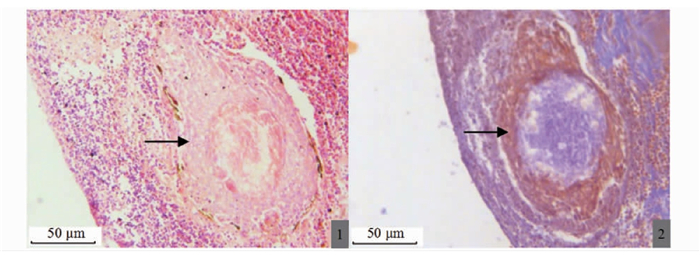
|
1.成熟肉芽组织H.E染色(→);2.脾脏内成熟肉芽肿组织纤维包膜染色(→)。 1. H.E staining of mature granulation tissue (→); 2. Fibrous envelope staining of mature granulomatous tissue in the spleen (→). 图版 Ⅲ 病鱼样品7号脾组织H.E和马松染色组织病理学观察 Plate Ⅲ Observation on histopathology of H.E and Masson staining of spleen tissue of sick fish sample No.7 |

|
1.病鱼样品4号肝组织肉芽肿内细菌分布(←所示为肉芽肿);2.6号脾组织肉芽肿内(I)、外(O)杆菌分布,M为肉芽肿包膜;3.7号肾组织肉芽肿内杆菌分布(←);4、5、6.4号、6号、7号杆菌分布图(100倍);7.6号H.E染色仅能看到肉芽肿(▲为组织内肉芽肿);8.6号抗酸染色准确显示病原菌滋生在肉芽肿内部(▲为组织内肉芽肿和大量滋生的病原菌)。 1. Bacterial distribution in granuloma in sick American shad No.4 liver(← granuloma shown); 2. No.6 for splenic tissue granuloma inside (I) and outer (O) distribution of bacteria, (M) is the granulomatous envelope; 3. Distribution of intracellular bacilli in renal granuloma in sick American shad No.7 (←); 4, 5, 6. Distribution of bacillus in sick American shad No.4, 6 and 7 (100 times); 7. H.E staining of sick American shad No.6 can only see granulomas (▲ is granuloma in the tissue); 8. Ziehl-Neelsen stain No.6 accurately shows that pathogenic bacteria breed in granuloma and grow inside granuloma (▲ is the granuloma in the tissue and a large number of pathogenic bacteria). 图版 Ⅳ 病鱼样品4、6和7号肝脏、肾脏和脾脏组织内滋生菌分布 Plate Ⅳ Distribution of proliferative bacteria in liver, kidney and spleen tissues of sick fish samples No.4, 6 and 7 |
肉芽肿形成的不同时期存在不同结构差异。形成早期,组织中无明显的肉芽肿结构,巨噬细胞和淋巴细胞持续性产生炎性介质,炎性细胞开始聚集,中心出现少量红细胞(图版Ⅱ-2); 形成中期,肉芽肿的形成逐渐成熟,具有典型的基本结构(图版Ⅱ-1)。大量的纤维细胞聚集逐渐形成纤维包膜,将部分病原菌包裹。肉芽肿的中心聚集的细菌,呈现较为明显的杆状(图版Ⅳ-4)。大量的杆菌集聚成团,高密度分布在肉芽肿中心,其间混合有坏死的组织细胞(图版Ⅳ-2)。炎性细胞与病原菌混合形成干酪性坏死,致使肉芽肿逐渐变大;形成后期,肉芽肿的外部形态较初成熟期更大,结构更加完整。肉芽肿外层的成纤维细胞转变为纤维细胞包膜,外层的包膜将坏死灶完全包裹(图版Ⅱ-2)。大量的炎性细胞在纤维包膜外聚集(图版Ⅱ-3, 4)。杆菌在组织间大量繁殖,除肉芽肿内大量聚集的菌体,包膜外仍有大量杆菌分布(图版Ⅳ-3), 病菌感染更加严重,分布的肉芽肿数量较初期明显增多;形成晚期,多个肉芽肿组织纤维包膜融合,形成由多个中心坏死灶组成的大型肉芽肿集团(图版Ⅱ-6)。坏死部位聚集的肉芽肿集团内部组成与形成中后期结构基本一致。同时在这些大型包膜的外侧也可见大量的嗜酸性粒细胞浸润(图版Ⅱ-6)。大量的肉芽肿组织占据了原有的组织空间,纤维包膜随之不断扩大。最后多个纤维包膜融合,形成肉眼可见的白色结节。
2.4 入侵器官组织肉芽肿内的病原菌确定抗酸染色显示,病鱼样品4号肝脏、脾脏和肾脏组织内均有大量紫红色菌团分布(图版Ⅳ-1, 3)。高倍镜下观察可见该病原菌为短杆菌(图版Ⅳ-4, 5, 6)。相比于病鱼样品6号肝组织H.E染色结果(图版Ⅳ-7), 抗酸染色能准确确定该病原菌分布在肉芽肿内部(图版Ⅳ-8)。
透射电镜结果证实该菌为短杆、无鞭毛的菌体,长度约为1.7~2.0 μm, 宽度约为0.3 μm(图版Ⅴ-1, 2)。肝脏、脾脏和肾脏细胞和细胞器均有不同程度的病变情况(图版Ⅴ-3, 5, 7), 亚显微观察见肝细胞内细胞器结构及细胞膜界限均不清晰,细胞内出现大量脂滴(图版Ⅴ-3); 胞内有菌体聚生产生(图版Ⅴ-4), 病菌侵入细胞质中,破坏组织原有结构(图版Ⅴ-1, 2)。亚显微观察见肾小管上皮细胞线粒体肿胀,部分空泡变性,嵴断裂,内质网收缩,溶酶体数量增加,细胞核核质不清晰,核膜界限不清(图版Ⅴ-7), 细胞结构被破坏(图版Ⅴ-8), 核仁溶解(图版Ⅴ-9)。胞内有菌体聚生产生(图版Ⅴ-4), 亚显微结构观察所示大量游离的红细胞分布在脾脏细胞间(图版Ⅴ-5)。同时可见吞噬细胞吞噬病原菌(图版Ⅴ-6)。除细菌外未见其他病毒颗粒和类立克次体等其他病原生物。
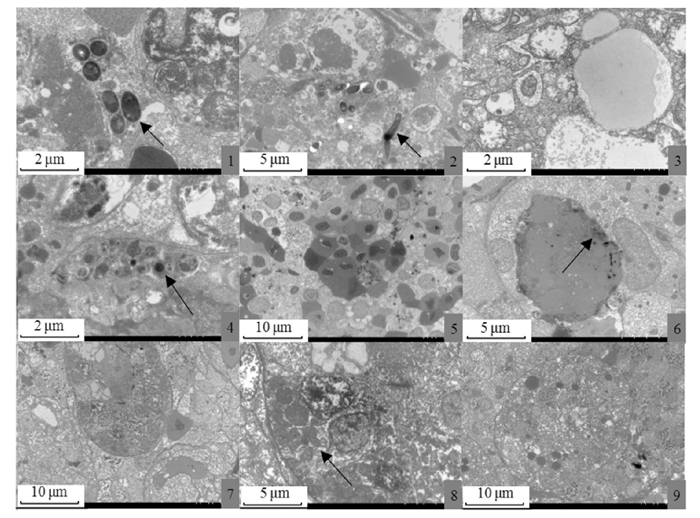
|
1、2.肝细胞间聚生菌体(↖);3.脂肪颗粒;4.脾细胞胞核附近聚生菌体(↖);5.脾细胞分布大量红细胞;6.巨噬细胞吞噬少量菌体(↗);7.肾脏细胞胞核内细胞器溶解,核膜界限不清;8.内质网收缩变形,溶酶体大量增多(↖);9.细胞核核仁溶解消失。 1, 2. Intercellular bacterial aggregation in the liver cells(↖); 3. Fat particles; 4. Consortium near the nucleus of spleen cells(↖); 5. Large number of free red blood cells in spleen cells; 6. Macrophages engulf a small amount of bacteria(↗); 7. Intracellular organelle dissolution in renal cells and the nuclear envelope was indistinct; 8. Endoplasmic reticulum contracted and deformed, the lysosomes increased in abundance (↖); 9. Nucleoli was dissolved and disappeared. 图版 Ⅴ 病鱼样品6号肝脏、肾脏和脾脏组织超微病理变化 Plate Ⅴ Ultrastructural pathological changes of liver, kidney and spleen tissues of sick fish sample No.6 |
本次研究中患病美国鲥鱼的临床症状为肝脏、肾脏和脾脏表面可见大量的白色结节,经组织切片观察确定白色结节为组织内形成的肉芽肿集团暴露器官表面部分。相关资料报道表明,大西洋鲑(Salmo salar)[20]、花鲈[6]、大黄鱼[21]、斑鳢(Channa maculata)[22]等感染诺卡氏菌的肉芽肿形成位置在心脏、脾脏、肾脏和肝脏,除上述感染部位外,在大口黑鲈(Micropterus salmoides)[23]的鳔中,斑鳢[22]的肌肉和卵巢内发现过肉芽肿组织以及虹鳟(Salmo gairdneri)[24]的脑中也出现过病理性结节。美国鲥鱼感染病原菌的病变主要集中在肝、脾和肾,其他器官组织未见肉芽肿分布,与上述研究结果存在差异。与乌鳢诺卡氏菌病[25]、 鱼诺卡氏菌感染卵形鲳鲹[26]和乌斑杂交鳢[27]的报道均不同,本实验研究中肉芽肿组织呈现典型的双层结构。患病的卵形鲳鲹和乌鳢组织内分布的肉芽肿在坏死灶和纤维组织包膜外还分布少量多核巨细胞和类上皮细胞[25, 27]。而在大口黑鲈结节病的研究中所报道的中层结构则为坏死的结缔组织和炎性细胞,外层则为多处稀疏的结缔组织和上皮细胞[28], 该肉芽肿组织结构与本研究也存在较大差异。而大黄鱼白点病[17, 29]、乌鳢诺卡氏菌病[5]的病理和超微病理的相关报道中则存在类似结构特征。除肝、肾和脾表面分布大量结节外,其他病症还包括体表鳞片下出血、腹腔积水、肝肿大淤血、肠道严重充血出血、脾脏发黑等症状,与鲈鱼和罗非鱼白点病[10, 30]病例中肠道严重充血、肾脏肿大、肝表面分布大量白斑存在不同病症特征。在大黄鱼内脏白点病的相关报道[31]中指出,患内脏白点病的大黄鱼病理表现为肝细胞崩解、肝细胞索消失,肝胰腺分离,窦状隙中大量炎性细胞浸润;肾小管上皮细胞肿胀,管腔闭塞;脾脏内大量炎性细胞浸润,含铁血黄素大量沉积[32], 与本研究中患病美国鲥鱼内脏结节病的病理特征表现存在差异。在美人鱼发光杆菌感染卵形鲳鲹的报道中,肝细胞肿大,肝血窦扩张,血管栓塞,肾小管上皮脱落、坏死,与基底膜分离形成空腔,大量炎性细胞浸润,脾血窦扩张淤血、间质出血[9], 与本研究结果肾组织病理变化相类似。本实验采用马松染色确定该肉芽肿的组织结构,比常规H.E染色[33]对该肉芽肿的包膜结构说明更清晰,并成为肉芽肿诊断的佐证。
鱼诺卡氏菌感染卵形鲳鲹[26]和乌斑杂交鳢[27]的报道均不同,本实验研究中肉芽肿组织呈现典型的双层结构。患病的卵形鲳鲹和乌鳢组织内分布的肉芽肿在坏死灶和纤维组织包膜外还分布少量多核巨细胞和类上皮细胞[25, 27]。而在大口黑鲈结节病的研究中所报道的中层结构则为坏死的结缔组织和炎性细胞,外层则为多处稀疏的结缔组织和上皮细胞[28], 该肉芽肿组织结构与本研究也存在较大差异。而大黄鱼白点病[17, 29]、乌鳢诺卡氏菌病[5]的病理和超微病理的相关报道中则存在类似结构特征。除肝、肾和脾表面分布大量结节外,其他病症还包括体表鳞片下出血、腹腔积水、肝肿大淤血、肠道严重充血出血、脾脏发黑等症状,与鲈鱼和罗非鱼白点病[10, 30]病例中肠道严重充血、肾脏肿大、肝表面分布大量白斑存在不同病症特征。在大黄鱼内脏白点病的相关报道[31]中指出,患内脏白点病的大黄鱼病理表现为肝细胞崩解、肝细胞索消失,肝胰腺分离,窦状隙中大量炎性细胞浸润;肾小管上皮细胞肿胀,管腔闭塞;脾脏内大量炎性细胞浸润,含铁血黄素大量沉积[32], 与本研究中患病美国鲥鱼内脏结节病的病理特征表现存在差异。在美人鱼发光杆菌感染卵形鲳鲹的报道中,肝细胞肿大,肝血窦扩张,血管栓塞,肾小管上皮脱落、坏死,与基底膜分离形成空腔,大量炎性细胞浸润,脾血窦扩张淤血、间质出血[9], 与本研究结果肾组织病理变化相类似。本实验采用马松染色确定该肉芽肿的组织结构,比常规H.E染色[33]对该肉芽肿的包膜结构说明更清晰,并成为肉芽肿诊断的佐证。
针对鱼类内脏结节病和结节症状的报道大多数集中在病原上,已见有杀香鱼假单胞菌(Pseudomonas plecoglossicida)[7, 34]、 鱼诺卡氏菌(Nocardia seriolae)[8, 26-27, 35-36]、美人鱼发光杆菌(Photobacterium damselae) [37-38]、舒伯特气单胞菌(Aeromonas schubertii)[18]、恶臭假单胞菌(P.putida)[33, 35, 39]、荧光假单胞菌(Pseudomonas fluorescens)[40-42]、嗜水气单胞菌(Aeromonas hydrophila)[10]、哈维氏弧菌(Vibrio harveyi)[43]、舒氏气单胞菌(Aeromonas schubertii)[44]及类立克次体[14-16]等病原相关报道,但未见在患病鱼的器官组织中定位说明,最新报道
鱼诺卡氏菌(Nocardia seriolae)[8, 26-27, 35-36]、美人鱼发光杆菌(Photobacterium damselae) [37-38]、舒伯特气单胞菌(Aeromonas schubertii)[18]、恶臭假单胞菌(P.putida)[33, 35, 39]、荧光假单胞菌(Pseudomonas fluorescens)[40-42]、嗜水气单胞菌(Aeromonas hydrophila)[10]、哈维氏弧菌(Vibrio harveyi)[43]、舒氏气单胞菌(Aeromonas schubertii)[44]及类立克次体[14-16]等病原相关报道,但未见在患病鱼的器官组织中定位说明,最新报道 鱼诺卡氏菌感染大口黑鲈[28]的研究结果没有明确定位肉芽肿中病原菌的存在。本研究通过对病鱼样品的肝、脾和肾3个组织进行抗酸染色,成功将分布在组织间肉芽肿中心的疑似致病菌清晰定位(紫红色短杆菌), 并通过亚显微结构观察也证实该疑似致病菌形态与抗酸染色结果一致。根据其形态、长度、抗酸性和无鞭毛等特征观察将其与舒氏气单胞菌(有鞭毛)[44]、美人鱼发光杆菌(长杆状)[37]、诺卡氏菌(弱抗酸)[26]等分枝状杆菌和类立克次体(圆形)[14-16]相区分。而有关该美国鲥鱼病原菌鉴定的相关研究有待进一步探索。
鱼诺卡氏菌感染大口黑鲈[28]的研究结果没有明确定位肉芽肿中病原菌的存在。本研究通过对病鱼样品的肝、脾和肾3个组织进行抗酸染色,成功将分布在组织间肉芽肿中心的疑似致病菌清晰定位(紫红色短杆菌), 并通过亚显微结构观察也证实该疑似致病菌形态与抗酸染色结果一致。根据其形态、长度、抗酸性和无鞭毛等特征观察将其与舒氏气单胞菌(有鞭毛)[44]、美人鱼发光杆菌(长杆状)[37]、诺卡氏菌(弱抗酸)[26]等分枝状杆菌和类立克次体(圆形)[14-16]相区分。而有关该美国鲥鱼病原菌鉴定的相关研究有待进一步探索。
该鱼场美国鲥鱼的养殖周期为14个月,发病期长达3个月,其间不断有患病鱼死亡,死亡的美国鲥鱼内脏肝、脾和肾表面均有结节分布。本实验中肝组织的病理结果与其他关于结节病的报道均存在不同[25, 33]。脾脏组织病变与乌鳢诺卡氏菌病[5]的研究内容相似。通过对接收病鱼样品的临床解剖,将器官病变程度轻重排序发现,泌尿系统的肾脏组织最先受到感染,也最为严重。这与卵形鲳鲹[26]感染 鱼诺卡氏菌后脾脏是最严重病变组织的报道不同。推测该致病菌最先通过血液循环进入肾脏,与乌鳢诺卡氏菌病[25]报道的肾脏是自然发病最早的病变器官相一致。随着病情的不断加重,肝脏、肾脏和脾脏的结节及其组织中肉芽肿数量的增多,体积增大,组织细胞变性坏死,各器官功能低下或衰竭,最终导致鱼体死亡。
鱼诺卡氏菌后脾脏是最严重病变组织的报道不同。推测该致病菌最先通过血液循环进入肾脏,与乌鳢诺卡氏菌病[25]报道的肾脏是自然发病最早的病变器官相一致。随着病情的不断加重,肝脏、肾脏和脾脏的结节及其组织中肉芽肿数量的增多,体积增大,组织细胞变性坏死,各器官功能低下或衰竭,最终导致鱼体死亡。
| [1] |
BRUMMETT A R, DUMONT J N. Kupffer's vesicle in Fundulus heteroclitus: a scanning and transmission electron microscope study[J]. Tissue and Cell, 1978, 10(1): 11-22. DOI:10.1016/0040-8166(78)90003-4 |
| [2] |
高小强, 洪磊, 刘志峰, 等. 美洲鲥仔稚鱼异速生长模式研究[J]. 水生生物学报, 2015, 39(3): 638-644. GAO X Q, HONG L, LIU Z F, et al. The study of allometric growth pattern of american shad larvae and juvenile (Alosa sapidissima)[J]. Acta Hydrobiologica Sinica, 2015, 39(3): 638-644. |
| [3] |
JIA Y J, CHEN Y F, GOUDIE C A, et al. Potential invasion risk of the introduced American shad Alosa sapidissima to aquatic ecosystem in China[J]. Acta Zoologica Sinica, 2007, 53(4): 625-629. |
| [4] |
高小强, 刘志峰, 黄滨, 等. 美洲鲥(Alosa sapidissima)胚胎发育形态学及组织切片观察[J]. 渔业科学进展, 2017, 38(5): 9-18. GAO X Q, LIU Z F, HUANG B, et al. Morphological and histological observation of the embryo of American Shad (Alosa sapidissima)[J]. Progress in Fishery Sciences, 2017, 38(5): 9-18. |
| [5] |
王桢, 葛明峰, 徐益军, 等. 乌鳢诺卡氏菌病的组织病理和超微病理观察[J]. 宁波大学学报(理工版), 2015, 28(2): 13-18. WANG Z, GE M F, XU Y J, et al. Histopathological and ultrapathological observation on Nocardiosis of Ophicephalus argus[J]. Journal of Ningbo University(NSEE), 2015, 28(2): 13-18. |
| [6] |
CHEN S C, LEE J L, LAI C C, et al. Nocardiosis in sea bass, Lateolabrax japonicus, in Taiwan[J]. Journal of Fish Diseases, 2000, 23(5): 299-307. DOI:10.1046/j.1365-2761.2000.00217.x |
| [7] |
ZHANG J T, ZHOU S M, AN S W, et al. Visceral granulomas in farmed large yellow croaker, Larimichthys crocea (Richardson), caused by a bacterial pathogen, Pseudomonas plecoglossicida[J]. Journal of Fish Diseases, 2014, 37(2): 113-121. DOI:10.1111/jfd.12075 |
| [8] |
TORANZO A E, BARREIRO S, CASAL J F, et al. Pasteurellosis in cultured gilthead seabream (Sparus aurata): first report in Spain[J]. Aquaculture, 1991, 99(1/2): 1-15. |
| [9] |
苏友禄, 冯娟, 郭志勋, 等. 美人鱼发光杆菌杀鱼亚种感染卵形鲳鲹的病理学观察[J]. 海洋科学, 2012, 36(2): 75-81. SU Y L, FENG J, GUO Z X, et al. Histopathological analysis of golden pompano Trachinotus ovatus infected with Photobacterium damselae subsp. piscicida[J]. Marine Sciences, 2012, 36(2): 75-81. |
| [10] |
金珊, 王国良, 赵青松, 等. 鲈细菌性类结节病的病原及血液病理研究[J]. 水产学报, 2004, 28(6): 703-708. JIN S, WANG G L, ZHAO Q S, et al. Pathogenic and hemopathological studies on the bacterial pseudotuberculosis of Lateolabrax japonicus[J]. Journal of Fisheries of China, 2004, 28(6): 703-708. |
| [11] |
黄郁葱, 简纪常, 吴灶和, 等. 卵形鲳鲹结节病病原的分离与鉴定[J]. 广东海洋大学学报, 2008, 28(4): 49-53. HUANG Y C, JIAN J C, WU Z H, et al. Isolation and identification of the pathogen causing Sarcoidosis of Trachinotus ovatus[J]. Journal of Guangdong Ocean University, 2008, 28(4): 49-53. DOI:10.3969/j.issn.1673-9159.2008.04.011 |
| [12] |
竹攸汀. 异育银鲫皮肤粘孢子虫病的病原和组织病理研究[D]. 上海: 上海海洋大学, 2012. ZHU Y T. Pathogen and histopathology studies of Myxosporean (Myxozoa: Myxosporea) infecting the skin of Allogynogenetic Crucian Carp (Carassius auratus gibelio)[D]. Shanghai: Shanghai Ocean University, 2012. |
| [13] |
耿毅, 汪开毓, 叶仕根, 等. 鲤一种粘孢子虫病的病理学研究[J]. 水产科学, 2003, 22(1): 11-13. GENG Y, WANG K Y, YE S G, et al. Study on the pathology of a new Myxosporidiosis in common carp[J]. Fisheries Science, 2003, 22(1): 11-13. DOI:10.3969/j.issn.1003-1111.2003.01.003 |
| [14] |
贾伟章, 孙晓凤, 郭琼林. 养殖乌鳢类立克次体分离纯化的初步研究[J]. 水生生物学报, 2004, 28(4): 448-449. JIA W Z, SUN X F, GUO Q L. Primary isolation and purification of Rickettsia-like Organism (RLO) from the tissues of Ophiocephalus argus[J]. Acta Hydrobiologica Sinica, 2004, 28(4): 448-449. DOI:10.3321/j.issn:1000-3207.2004.04.021 |
| [15] |
郭琼林, 贾伟章, 韩先朴, 等. 我国淡水养殖乌鳢的类立克次体感染[J]. 自然科学进展, 2004, 14(1): 59-63. GUO Q L, JIA W Z, HAN X P, et al. Rickettsia-like Organism infection of freshwater aquaculture in China[J]. Progress in Natural Science, 2004, 14(1): 59-63. DOI:10.3321/j.issn:1002-008X.2004.01.010 |
| [16] |
郭琼林, 孙晓凤, 贾伟章, 等. 养殖乌鳢类立克次体感染的超微病理学研究[J]. 水生生物学报, 2007, 31(2): 265-271. GUO Q L, SUN X F, JIA W Z, et al. Ultrastructural pathological studies on rickettsia-like organism infection in a cultured fish, Ophiocephalus argus[J]. Acta Hydrobiologica Sinica, 2007, 31(2): 265-271. DOI:10.3321/j.issn:1000-3207.2007.02.019 |
| [17] |
张丹枫, 安树伟, 周素明, 等. 大黄鱼(Pseudosciaena crocea)内脏白点病的组织病理和超微病理分析[J]. 渔业科学进展, 2017, 38(4): 11-16. ZHANG D F, AN S W, ZHOU S M, et al. Histopathology and ultrastructure of visceral white-spots in Pseudosciaena crocea[J]. Progress in Fishery Sciences, 2017, 38(4): 11-16. |
| [18] |
常藕琴, 石存斌, 任燕, 等. 高体类结节病的病理组织学观察[J]. 水产学报, 2013, 37(6): 936-943. CHANG O Q, SHI C B, REN Y, et al. Histopathological observations on Pseudotuberculosis of Seriola dumerili[J]. Journal of Fisheries of China, 2013, 37(6): 936-943. |
| [19] |
龙波, 王均, 贺扬, 等. 加州鲈源维氏气单胞菌的分离、鉴定及致病性[J]. 中国兽医学报, 2016, 36(1): 48-55. LONG B, WANG J, HE Y, et al. Isolation, identification and pathogenicity of Aeromonas veronii isolated from Micropterus salmoides[J]. Chinese Journal of Veterinary Science, 2016, 36(1): 48-55. |
| [20] |
BRANSDEN M P, CARSON J, MUNDAY B L, et al. Nocardiosis in tank‐reared Atlantic salmon, Salmo salar L.[J]. Journal of Fish Diseases, 2000, 23(1): 83-85. DOI:10.1046/j.1365-2761.2000.00201.x |
| [21] |
WANG G L, YUAN S P, JIN S. Nocardiosis in large yellow croaker, Larimichthys crocea (Richardson)[J]. Journal of Fish Diseases, 2005, 28(6): 339-345. DOI:10.1111/j.1365-2761.2005.00637.x |
| [22] |
CHEN S C, TUNG M C, TSAI W C. An epizootic in Formosa snake-head fish, Channa maculata Lacepede, caused by Nocardia asteroides in fresh water pond in southern Taiwan[J]. COA Fisheries Series, 1989, 15: 42-48. |
| [23] |
LAI K C, CHEN S N, KOU G H. Studies on the infection of Nocardia sp. in cultured largemouth bass (Micropterus salmoides, Lacepede)[J]. COA Fisheries Series, 1991(29): 1-15. |
| [24] |
SNIESZKO S F, BULLOCK G L, DUNBAR C E, et al. Nocardial infection in hatchery-reared fingerling rainbow trout (Salmo gairdneri)[J]. Journal of Bacteriology, 1964, 88(6): 1809-1810. DOI:10.1128/JB.88.6.1809-1810.1964 |
| [25] |
常藕琴, 石存斌, 潘厚军, 等. 乌鳢诺卡氏菌病的组织病理学[J]. 水产学报, 2008, 32(2): 209-216. CHANG O Q, SHI C B, PAN H J, et al. Histopathological study on nocardiosis of Ophicephalus argus[J]. Journal of Fisheries of China, 2008, 32(2): 209-216. |
| [26] |
满其蒙, 徐力文, 区又君, 等. 鱼诺卡氏菌感染卵形鲳鲹的组织病理学研究[J]. 广东农业科学, 2012, 39(21): 132-135. MAN Q M, XU L W, QU Y J, et al. Histopathological study on golden pompano Trachinotus ovatus infected with Nocardia seriolae[J]. Guangdong Agricultural Sciences, 2012, 39(21): 132-135. DOI:10.3969/j.issn.1004-874X.2012.21.042 |
| [27] |
王文基, 陈建林, 侯素莹, 等. 鱼诺卡氏菌感染乌斑杂交鳢的组织病理学研究[J]. 基因组学与应用生物学, 2019, 38(10): 4439-4446. WANG W J, CHEN J L, HOU S Y, et al. A histopathological study of Nocardia seriolae infection in hybrid snakehead[J]. Genomics and Applied Biology, 2019, 38(10): 4439-4446. |
| [28] |
何晟毓, 魏文燕, 刘韬, 等. 大口黑鲈致死性结节病病原的分离、鉴定及组织病理学观察[J]. 水产学报, 2020, 44(2): 253-265. HE S Y, WEI W Y, LIU T, et al. Isolation, identification and histopathological study on lethal sarcoidosis of Micropterus salmoides[J]. Journal of Fisheries of China, 2020, 44(2): 253-265. |
| [29] |
安树伟. 大黄鱼内脏白点病的病原及组织病理研究[D]. 宁波: 宁波大学, 2013. AN S W. Identification of causative pathogen for visceral white-spot and histopathologic research on Pseudosciaena crocea[D]. Ningbo: Ningbo University, 2013. |
| [30] |
梅广海, 朱永生, 曹榕, 等. 越冬罗非鱼细菌性类结节病的初步研究[J]. 水产科学, 2002, 21(4): 9-11. MEI G H, ZHU Y S, CAO R, et al. A preliminary study on the bacterial pseudotuberculosis of wintering tilapia[J]. Fisheries Science, 2002, 21(4): 9-11. |
| [31] |
金珊, 王国良, 赵青松, 等. 海水网箱养殖大黄鱼弧菌病的流行病学研究[J]. 水产科学, 2005, 24(1): 17-19. JIN S, WANG G L, ZHAO Q S, et al. Epidemiology of vibriosis in large yellow croaker Pseudosciaena crocea (Richardson) in marine cage culture[J]. Fisheries Science, 2005, 24(1): 17-19. DOI:10.3969/j.issn.1003-1111.2005.01.005 |
| [32] |
邱杨玉, 郑磊, 毛芝娟, 等. 大黄鱼(Larimichthys crocea)内脏白点病的病原分离和组织病理学观察[J]. 微生物学通报, 2012, 39(3): 361-370. QIU Y Y, ZHENG L, MAO Z J, et al. Isolation and identification of the causative agent and histopathology observation of white-spots disease in internal organs of Larimichthys crocea[J]. Microbiology China, 2012, 39(3): 361-370. |
| [33] |
许斌福, 程海华, 池洪树, 等. 大黄鱼内脏白点病的病原分析与鉴定[J]. 福建农业学报, 2015, 30(7): 631-635. XU B F, CHENG H H, CHI H S, et al. Identification of pathogen responsible for white-spots disease in Larimichthys crocea[J]. Fujian Journal of Agricultural Sciences, 2015, 30(7): 631-635. DOI:10.3969/j.issn.1008-0384.2015.07.002 |
| [34] |
张杰, 毛芝娟. 大黄鱼内脏白点病病原杀香鱼假单胞菌及其毒力因子研究进展[J]. 浙江万里学院学报, 2015, 28(6): 69-76, 81. ZHANG J, MAO Z J. Research progress on pseudomonas plecoglossicida and its virulence factors of large yellow croaker (Pseudosciaena crocea)[J]. Journal of Zhejiang Wanli University, 2015, 28(6): 69-76, 81. DOI:10.3969/j.issn.1671-2250.2015.06.013 |
| [35] |
王瑞旋, 刘广锋, 王江勇, 等. 养殖卵形鲳鲹诺卡氏菌病的研究[J]. 海洋湖沼通报, 2010(1): 52-58. WANG R X, LIU G F, WANG J Y, et al. Studies on Nocardiasis infected farming Trachinotus ovatus[J]. Transactions of Oceanology and Limnology, 2010(1): 52-58. DOI:10.3969/j.issn.1003-6482.2010.01.008 |
| [36] |
王国良, 徐益军, 金珊. 乌鳢致病诺卡氏菌的鉴定及系统发育分析[J]. 水产学报, 2008, 32(3): 449-454. WANG G L, XU Y J, JIN S. Identification and phylogenetic analyses of Nocardia in snakehead, Ophiocephalus argus Cantor[J]. Journal of Fisheries of China, 2008, 32(3): 449-454. |
| [37] |
王二龙, 汪开毓, 陈德芳, 等. 养殖乌鳢内脏结节病的病原分离、鉴定与药物敏感性分析[J]. 华中农业大学学报, 2015, 34(5): 90-98. WANG E L, WANG K Y, CHEN D F, et al. Isolation, identification and drug sensitivity test of causative pathogen offarmed snakehead (Channa argus)with visceral sarcoidosis[J]. Journal of Huazhong Agricultural University, 2015, 34(5): 90-98. |
| [38] |
NAGANO I, INOUE S, KAWAI K, et al. Repeatable immersion infection with Photobacterium damselae subsp. piscicida reproducing clinical signs and moderate mortality[J]. Fisheries Science, 2009, 75(3): 707-714. DOI:10.1007/s12562-009-0099-8 |
| [39] |
刘春, 李凯彬, 王庆, 等. 杂交鳢(斑鳢♀×乌鳢♂)内脏类结节病病原菌的分离、鉴定与特性分析[J]. 水产学报, 2012, 36(7): 1119-1125. LIU C, LI K B, WANG Q, et al. Isolation, identification and characterization of Aeromonas schubertii from hybrid snakehead(Channa maculata ♀ × C. argus ♂)[J]. Journal of Fisheries of China, 2012, 36(7): 1119-1125. |
| [40] |
BALBOA S, FERGUSON H W, ROMALDE J L. Phenotypic, serological and genetic characterization of Pseudomonas anguilliseptica strains isolated from cod, Gadus morhua L., in northern Europe[J]. Journal of Fish Diseases, 2007, 30(11): 657-664. DOI:10.1111/j.1365-2761.2007.00849.x |
| [41] |
BLANCO M M, GIBELLO A, VELA A I, et al. PCR detection and PFGE DNA macrorestriction analyses of clinical isolates of Pseudomonas anguilliseptica from winter disease outbreaks in sea bream Sparus aurata[J]. Diseases of Aquatic Organisms, 2002, 50(1): 19-27. |
| [42] |
LÓPEZ-ROMALDE S, MAGARIÑOS B, RAVELO C, et al. Existence of two O-serotypes in the fish pathogen Pseudomonas anguilliseptica[J]. Veterinary Microbiology, 2003, 94(4): 325-333. DOI:10.1016/S0378-1135(03)00124-X |
| [43] |
张静. 网箱鲈鱼内脏白点病病原鉴定及两种快速检测方法的建立[D]. 舟山: 浙江海洋学院, 2010. ZHANG J. Isolation and identification of causative pathogen for visceral white spot in Lateolabrax japonicus stocked in sea-cage and establishment of two rapid detection methods[D]. Zhoushan: Zhejiang Ocean University, 2012. |
| [44] |
罗霞, 邓国成, 廖国礼, 等. 斑鳢内脏白点病病原的分离鉴定[J]. 大连海洋大学学报, 2012, 27(2): 95-100. LUO X, DENG G C, LIAO G L, et al. Isolation and identification of causative pathogen for visceral white spot in Taiwan snakehead Channa maculata[J]. Journal of Dalian Ocean University, 2012, 27(2): 95-100. |
2. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China;
3. Key Laboratory of Freshwater Aquatic Genetic Resources, Ministry of Agriculture and Rural Affairs, Shanghai Ocean University, Shanghai 201306, China;
4. Shanghai Qinmiao Biological Technology Co., Ltd., Shanghai 201802, China
 2021,
Vol. 30
2021,
Vol. 30


