骨形态发生蛋白(BMPs)是一组二聚肽分子,是转化生长因子-β(Transforming growth factor-β superfamily)超家族中最大的亚家族[1]。迄今,已有20多种BMPs被确认,其中GDF9和BMP15是BMPs家族中的重要成员,两者具有很高的同源性。GDF9和BMP15主要由卵母细胞分泌,并主要通过结合Ⅰ型和Ⅱ型两种Ser/Thr激酶受体进行信号转导,发挥调节卵巢发育等生物学功能[2]。GDF9与BMP15的Ⅱ型受体基因均为BMPR2,但GDF9的Ⅰ型受体基因为TGF-βR1(激活素受体样激酶5,ALK5),BMP15的Ⅰ型受体基因则为BMPR1B (ALK6) [3, 4]。
近年来,有关GDF9与BMP15在鱼类卵细胞发育与成熟过程中的表达研究已取得了一定的进展。在欧洲海鲈(Dicentrachus labrax) [5, 6]、鳗鲡(Anguilla australis)[7]、黄鳝(Monopterus albus)[8]、虹鳟(Oncorhynchus mykiss) [9]、斑马鱼(Danio rerio)[10, 11]和异育银鲫(Carassius auratus gibelio)[12, 13]等鱼类中,GDF9与BMP15 mRNA水平随卵细胞发育而降低,但BMP15 mRNA水平在斑马鱼不同发育阶段卵细胞中无显著差异。在武昌鱼(Megalobrama amblycephala)中,GDF9 mRNA在卵黄发生前期表达量降低,但在成熟期表达量升高[14]。这些研究提示GDF9与BMP15在硬骨鱼类卵细胞发育过程中发挥重要调控作用。值得注意的是,BMPs调控机体生物学功能不仅发生于配体水平,在受体水平也存在一定的影响。在斑马鱼中证实,GDF9与BMP15Ⅱ型受体基因(bmpr2a与bmpr2b) mRNA水平随卵细胞发育而发生改变[15]。斑马鱼BMPR1B(Alk6b)受体基因突变可损害生殖细胞分化并可造成生殖细胞瘤[16]。因此,本实验拟利用实时荧光定量PCR分析GDF9和BMP15受体基因在异育银鲫卵细胞发育过程中的表达和定位,旨在为阐明硬骨鱼类GDF9与BMP15信号转导途径以及其对下游目的基因的分子调控方式提供参考资料。
1 材料与方法 1.1 实验材料性成熟的异育银鲫购自上海奉城水产批发市场。解剖异育银鲫后取出部分精巢、卵巢、脑、鳃、肾脏、肝胰脏和肌肉。将卵巢放于直径10 cm内含有金鱼任氏液的培养皿中,采用机械分离法获得不同发育时期的卵细胞。根据直径大小对异育银鲫的卵细胞进行分类,即Ⅰ期(0.025~0.062 mm)、Ⅱ期(0.08~0.16 mm)、Ⅲ1期(0.22~0.36 mm)、Ⅲ2期(0.40~0.625 mm)和Ⅳ期(0.9~1.2 mm)[17]。采用冷刺激法分离卵母细胞和滤泡细胞,即将Ⅳ期卵细胞在4 ℃放置30 min,然后在不损伤卵母细胞的情况下,用解剖针将滤泡细胞与卵母细胞进行分离[10]。所获得各样品于液氮中速冻,-80 ℃保存用于总RNA的提取。
1.2 总RNA的提取及cDNA合成将样品按RNAiso(TaKaRa)试剂说明书进行总RNA提取,DNase去除DNA污染,加入适量RNase-free Water溶解RNA后加入RNA Inhibitor,于-80 ℃保存。琼脂糖凝胶电泳检测RNA完整性,紫外分光光度法分析RNA浓度与质量。在PrimeScript Reverse Transcriptase (TaKaRa)和1 μL Oligo(dT)30引物作用下合成cDNA,将反转录产物于-20 ℃保存。
1.3 Real-Time实时荧光定量PCR根据本实验室获得的GDF9与BMP15各受体基因序列(GenBank No. JX024254- JX024259),应用primer 5.0软件设计RT-PCR及实时荧光定量PCR的引物,具体序列见表1。以β-actin为内参基因,分析各受体基因的相对表达水平。
| 表1 GDF9与BMP15Ⅰ型与Ⅱ型受体基因所用引物 Tab.1 The primers for expression of GDF9 and BMP15 TypeⅠand TypeⅡreceptor genes |
Real-Time实时荧光定量PCR参照LIU等[10]的方法。PCR反应体系20 μL ,包括SYBR premix Ex Taq (TaKaRa) 10 μL,5倍稀释的cDNA模板 2 μL,上下游引物各0.4 μL,ddH2O 7.2 μL。PCR反应条件为95 ℃ 30 s;94 ℃ 5 s,60 ℃ 30 s,40个循环。PCR反应结束后,对扩增产物进行熔解曲线分析,检测反应特异性,采用2-△△Ct法分析各受体基因的相对表达量。
1.4 数据处理与分析文中数据采用SPSS 17.0进行统计处理,利用one-way方差分析进行差异显著性检验,显著水平为P<0.05,实验结果X±SE表示。用GraphPad Prism 6.0软件作图。
2 结果 2.1 异育银鲫GDF9与BMP15Ⅰ型与Ⅱ型受体基因在卵巢中的表达利用实时荧光定量PCR法,本实验研究了6个受体基因在卵巢不同时期卵细胞中表达水平的变化规律。结果表明,随着卵细胞的发育,TGF-βR1a mRNA表达水平呈现出先降低再升高的趋势,其在Ⅲ1期时降到最低(P<0.05),并在Ⅲ1期至Ⅳ期的发育过程中逐渐升高(P<0.05)(图1a)。TGF-βR1b在Ⅰ、Ⅱ期卵细胞中表达较低,当卵细胞发育至Ⅲ1期时,其表达水平显著升高,并持续至卵细胞发育后期(P<0.05,图1a)。BMPR1Ba与BMPR1Bb基因在卵细胞发育过程中的表达规律相似,为先降低后升高,其在Ⅲ1期卵细胞中表达量降至最低,并随后显著升高(P<0.05,图1b)。BMPR2a与BMPR2b基因在Ⅰ期卵细胞中表达最高,随着卵细胞的发育其表达逐渐降低,BMPR2b变化趋势较缓,而BMPR2a则较强(P<0.05,图1c)。
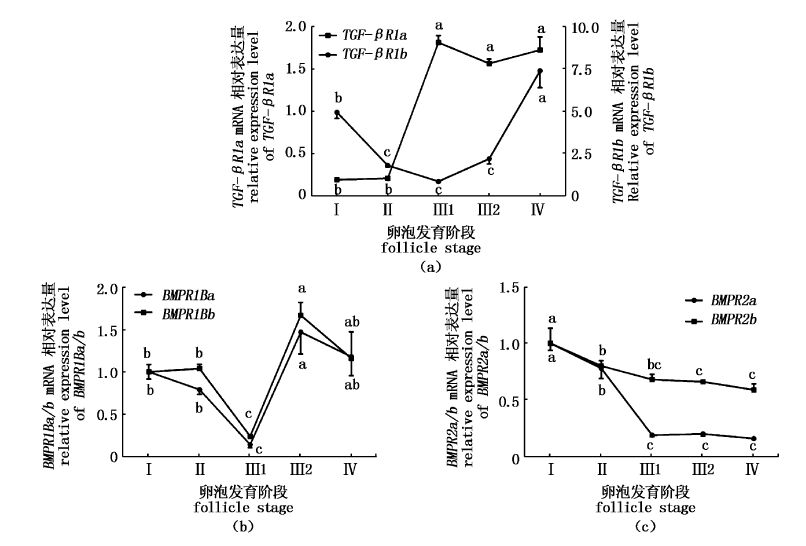
|
图1
异育银鲫TGF-βR1a、TGF-βR1b、BMPR1Ba、BMPR1Bb、BMPR2a及BMPR2b基因在各期卵细胞中的表达
Fig.1
The expression level of TGF-βR1a,TGF-βR1b,BMPR1Ba,BMPR1Bb,BMPR2a and BMPR2b mRNA in different follicles developing stages of gibel carp
A. TGF-βR1a和TGF-βR1b基因; B. BMPR1Ba和BMPR1Bb基因; C. BMPR2a和BMPR2b基因。不同字母表示相应组间差异达到显著水平(P<0.05)。样品结果用平均值±标准误来表示。 A. TGF-βR1a and TGF-βR1b genes; B. BMPR1Ba and BMPR1Bb genes; C. BMPR2a and BMPR2b genes. Different letters indicate statistical significance (P<0.05). The results were shown as mean ± SE. |
为了进一步明确GDF9与BMP15受体基因在卵细胞中的表达特性,本实验测定了6个受体基因在Ⅳ期卵细胞的卵母细胞与滤泡细胞中的表达情况。结果如图2所示,TGF-βR1b、BMPR1Bb、BMPR2a及BMPR2b mRNA在滤泡细胞中表达水平显著高于各卵母细胞中的表达水平(P<0.05),而TGF-βR1a与BMPR1Ba基因则在卵母细胞与滤泡细胞中均有表达。
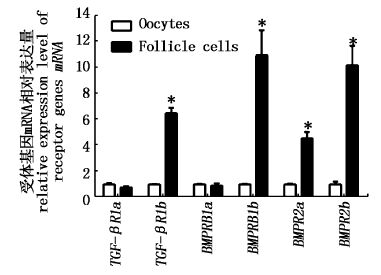
|
图2
异育银鲫TGF-βR1a、TGF-βR1b、BMPR1Ba、BMPR1Bb、BMPR2a及BMPR2b基因mRNA在卵母细胞与滤泡细胞中的表达
Fig.2
The expression level of TGF-βR1a,TGF-βR1b,BMPR1Ba,BMPR1Bb,BMPR2a and BMPR2b mRNA in oocytes and follicle cells of gibel carp
*”代表两组之间差异达到显著水平(P<0.05)。样品结果以平均值±标准误表示。 The “*” indicates statistical significance (P<0.05) between the two groups. The results were shown as mean ± SE. |
在哺乳动物中已证实,GDF9与BMP15可通过BMPs-Receopter-Smads途径调控卵细胞发育。如在人腔前卵泡发育过程中,ALK5(TGFβR1)mRNA随卵泡发育呈先上升后降低的变化趋势,ALK6(BMPR1B)在原始卵泡阶段表达水平较低,随后无明显变化,但BMPR2 mRNA在原始卵泡发育阶段即高水平表达并随着卵泡的发育表达水平逐渐增加[18]。本实验结果表明在异育银鲫卵细胞发育过程中,TGF-βR1a、BMPR1Ba与BMPR1Bb mRNA呈现出先降低再升高的趋势,TGF-βR1b表达水平在Ⅱ期卵细胞后显著增高。TGF-βR1a与TGF-βR1b在卵细胞发育过程中存在明显不同,这提示在卵细胞发育后期GDF9可能通过与TGF-βR1a结合发挥其生物学功能。GDF9与BMP15Ⅰ型受体基因的表达变化趋势不同,揭示两者在异育银鲫卵细胞发育不同阶段可能发挥不同功能。在异育银鲫卵细胞发育过程中,GDF9与BMP15在Ⅰ期卵细胞中表达量最高,并随着卵细胞的发育逐渐降低[12, 13]。本研究表明,当卵细胞由Ⅰ期发育至Ⅱ期时,BMPR2a/b的表达显著降低,随着卵细胞继续发育,两者表达进一步显著降低。这与GDF9与BMP15在异育银鲫不同发育阶段卵细胞中的表达变化趋势相似,这进一步提示BMPs通过受体介导在鱼类卵细胞发育初期发挥着重要功能。以上结果也进一步证实了在鱼类中GDF9与BMP15通过BMPR2a/b受体进行转导的信号通路,在卵细胞发育过程中发挥着重要调控作用。在哺乳动物卵巢中,促性腺激素与BMP亚家族之间存在相互作用。促性腺激素FSH能促进内源性BMP的信号转导,增强人颗粒细胞中BMPⅠ型及Ⅱ型受体基因的表达[19]。研究已证实,hCG诱发异育银鲫卵母细胞体外成熟过程中,GDF9与BMP15 mRNA水平呈显著变化[12, 13]。在异育银鲫卵细胞发育与成熟过程中,促性腺激素与BMPs各受体基因之间是否存在联系还需进一步研究。
在硬骨鱼类的卵细胞发生、发育和成熟这一极其复杂的动态生理过程中,来自下丘脑-垂体-性腺轴分泌的促性腺激素和卵细胞自身分泌的BMPs、Kit配体、激活素等因子,通过内分泌、旁分泌和自分泌等多种途径的共同作用使其有序进行,其中卵母细胞和周围滤泡细胞之间的双向通讯对卵细胞发育、成熟起着关键作用[20, 21, 22]。来源于由卵母细胞分泌的GDF9和BMP15可作用于邻近的颗粒细胞,调控激活素/抑制素的表达和抑制素的分泌[21]。在绵羊中,BMPRIB在卵母细胞和颗粒细胞中表达,但BMPR2除了在卵母细胞和颗粒细胞中可检测到外,在膜细胞中也可检测到其表达[23]。与绵羊BMPR2相似,猪BMPR1A、BMP1B和BMPR2也在卵母细胞、颗粒细胞和膜细胞中均有表达[24]。在斑马鱼中证实,BMPR2A和BMPR2B主要在滤泡细胞表达,提示BMPs可以通过旁分泌方式调控滤泡细胞的功能[15]。因此,研究GDF9与BMP15受体基因在卵母细胞与滤泡细胞中的表达是十分必要的。本实验结果表明,TGF-βR1b、BMPR1Bb、BMPR2a及BMPR2b基因主要存在于滤泡细胞中,该结果与斑马鱼中的结果较为一致[15]。多种激素与局部旁分泌因子可与滤泡细胞中的受体结合,进而调节卵细胞发育与成熟。因此,本实验的结果进一步证明GDF9和BMP15可与滤泡细胞中的受体基因TGF-βR1b、BMPR1Bb、BMPR2相结合,形成卵细胞内的复杂调控网络,调节卵细胞中卵母细胞与滤泡细胞间的信号传递。
| [1] | HELDIN C H, MIYAZONO K, TEN DIJKE P. TGF-β signalling from cell membrane to nucleus through SMAD proteins[J]. Nature, 1997, 390(6659):465-471. |
| [2] | BONDESTAM J, KAIVO-OJA N, KALLIO J, et al. Engagement of activin and bone morphogenetic protein signaling pathway Smad proteins in the induction of inhibin B production in ovarian granulosa cells[J]. Molecular and Cellular Endocrinology, 2002, 195(1/2):79-88. |
| [3] | MAZERBOURG S, KLEIN C, ROH J, et al. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5[J]. Molecular Endocrinology,2004,18(3):653-665. |
| [4] | JUENGEL J L,MCNATTY K P. The role of proteins of the transforming growth factor-β superfamily in the intraovarian regulation of follicular development[J]. Human Reproduction Update, 2005, 11(2):144-161. |
| [5] | HALM S, IBAŇEZAJ, TYLERCR, et al. Molecular characterisation of growth differentiation factor 9(GDF9) and bone morphogenetic protein 15(bmp15) and their patterns of gene expression during the ovarian reproductive cycle in the European sea bass[J]. Molecular and Cellular Endocrinology, 2008, 291(1/2):95-103. |
| [6] | GARCÍA-ÓPEZ Á, SÁNCHEZ-AMAYA MI, HALM S, et al. Bone morphogenetic protein 15 and growth differentiation factor 9 expression in the ovary of European Sea bass(Dicentrarchus labrax): Cellular localization, developmental profiles, and response to unilateral ovariectomy[J]. General and Comparative Endocrinology, 2011, 174(3): 326-334. |
| [7] | LOKMAN P M, KAZETO Y, OZAKI Y, et al. Effects of reproductive stage, GH, and 11-ketotestosterone on expression of growth differentiation factor-9 in the ovary of the eel, Anguilla australis[J]. Reproduction,2010,139(1): 71-83. |
| [8] | HE Z, WU Y S, XIE J, et al. Growth differentiation factor 9(Gdf9) was localized in the female as well as male germ cells in a protogynous hermaphroditic teleost fish, ricefield eel Monopterus albus[J]. General and Comparative Endocrinology, 2012,178(2):355-362. |
| [9] | LANKFORD S E, WEBER G M. Temporal mRNA expression of transforming growth factor-beta superfamily members and inhibitors in the developing rainbow trout ovary[J]. General and Comparative Endocrinology, 2010, 166(2): 250-258. |
| [10] | LIU L, GE W. Growth differentiation factor 9 and its spatiotemporal expression and regulation in the zebrafish ovary[J]. Biology of Reproduction, 2007,76(2):294-302. |
| [11] | CLELLAND E, KOHLI G, CAMPBELL R K, et al. Bone morphogenetic protein-15 in the zebrafish ovary: complementary deoxyribonucleic acid cloning, genomic organization, tissue distribution, and role in oocyte maturation[J]. Endocrinology, 2006, 147(1): 201-209. |
| [12] | CHEN A Q, LIU Z W, YANG Z G, et al. Characterization of bmp15 and its regulation by human chorionic gonadotropin in the follicle of gibel carp(Carassius auratus gibelio) [J]. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology, 2012, 163(1):121-128. |
| [13] | LIU Z W, CHEN A Q, YANG Z G, et al. Molecular characterization of growth differentiation factor 9 and its spatio-temporal expression pattern in gibel carp(Carassius auratus gibelio)[J]. Molecular Biology Reports, 2012, 39(4):3863-3870. |
| [14] | HUANG C X, WEI X L, CHEN N, et al. Growth differentiation factor 9 of Megalobrama amblycephala: molecular characterization and expression analysis during the development of early embryos and growing ovaries[J]. Fish Physiology and Biochemistry, 2014, 40(1): 193-203. |
| [15] | LI C W, GE W. Spatiotemporal expression of bone morphogenetic protein family ligands and receptors in the zebrafish ovary: a potential paracrine-signaling mechanism for oocyte-follicle cell communication[J]. Biology of Reproduction, 2011, 85(5): 977-986. |
| [16] | NEUMANN J C, CHANDLER G L, DAMOULIS V A, et al. Mutation in the type IB bone morphogenetic protein receptor Alk6b impairs germ-cell differentiation and causes germ-cell tumors in zebrafish[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(32): 13153-13158. |
| [17] | 侯亚义, 袁传宓. 异育银鲫的卵细胞发生[J]. 南京师范大学学报:自然科学版, 1989, 12(1): 66-72. HOU Y Y, YUAN C F. Studies of the growing ocytes of carassius auratus gibelio(Bloch)[J]. Journal of Nanjing Normal University: Natural Science, 1989, 12(1): 66-72. |
| [18] | KRISTENSEN S G, ANDERSEN K, CLEMENT C A, et al. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries[J]. Molecular Human Reproduction, 2014, 20(4): 293-308. |
| [19] | MIYOSHI T, OTSUKA F, SUZUKI J, et al. Mutual regulation of follicle-stimulating hormone signaling and bone morphogenetic protein system in human granulosa cells[J]. Biology of Reproduction, 2006, 74(6): 1073-1082. |
| [20] | 贾玉东, 雷霁霖. 硬骨鱼类卵子质量研究进展[J]. 中国水产科学, 2012, 19(3): 545-555. JIA Y D, LEI J L. Advances in teleost egg quality research[J]. Journal of Fishery Sciences of China, 2012, 19(3): 545-555. |
| [21] | LI G W, GE W. Regulation of the activin-inhibin-follistatin system by bone morphogenetic proteins in the zebrafish ovary[J]. Biology of Reproduction, 2013, 89(3): 55, 1-10. |
| [22] | YAO K, LAU S W, GE W. Differential regulation of Kit ligand A expression in the ovary by IGF-I via different pathways[J]. Molecular Endocrinology, 2014, 28(1): 138-150. |
| [23] | WILSON T, WU X Y, JUENGEL J L, et al. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor(ALK-6) that is expressed in both oocytes and granulosa cells[J]. Biology of Reproduction, 2001, 64(4): 1225-1235. |
| [24] | PARADIS F, NOVAK S, MURDOCH G K, et al. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig[J]. Reproduction, 2009, 138(1): 115-129. |
 2015, Vol. 24
2015, Vol. 24


