2. 江西天人生态股份有限公司, 江西 吉安 343100;
3. 中国农业大学 植物保护学院, 北京 100193
2. Jiangxi Tianren Ecological Co. Ltd., Ji'an 343100, Jiangxi Province, China;
3. College of Plant Protection, China Agricultural University, Beijing 100193, China
由胶孢炭疽菌Colletotrichum gloeosporioides和炭疽菌C. aenigma (异名:C. populi)引起的杨树炭疽病是杨树的重要病害之一[1-3],主要侵染枝、叶,导致杨树叶片枯死,可为害毛白杨、北京杨、箭杆杨、小叶杨、银白杨、加杨、青杨、黑杨及钻天杨等多种杨树[1-2]。该病曾先后在中国北京、河南、河北、山东、陕西、宁夏、新疆等地区发生[4],尤以毛白杨和北京杨受害最为严重[1],2005年北京市延庆地区北京杨的病株率甚至达到了100%[5],且近年来在各地发病呈上升趋势[4, 6]。此外,杨树炭疽病菌还可能威胁到其他相邻的寄主植物,如引起苹果、刺槐等发生炭疽病[7]。因此,有效控制杨树炭疽病已成为杨树产业中亟待解决的问题[8]。
目前生产上对炭疽病仍以化学防治为主,常用药剂有保护性杀菌剂(铜制剂等)和内吸性杀菌剂(苯并咪唑类、甲氧基丙烯酸酯类、麦角甾醇生物合成脱甲基酶抑制剂等),其中以苯并咪唑类(MBCs)的多菌灵和麦角甾醇生物合成脱甲基酶抑制剂类(DMIs)应用最为广泛[9-11]。然而,长期大量使用同类型杀菌剂,容易使病原菌产生抗药性,影响防治效果[12-13]。已有研究表明,来自多种寄主上的炭疽菌对MBCs和DMIs等多种杀菌剂产生了抗药性[14-17]。但目前尚未见使用这两类药剂防治杨树炭疽病的报道。因此,本研究测定了引起北京地区杨树炭疽病的59株胶孢炭疽菌和4株炭疽菌对MBCs杀菌剂多菌灵和3种DMIs杀菌剂(苯醚甲环唑、戊唑醇、三唑酮)的敏感性,分析了59株胶孢炭疽菌的敏感性频率分布,并进一步比较了不同寄主来源胶孢炭疽菌的敏感性差异以及对4种供试杀菌剂敏感性之间的相关性,以期明确北京地区杨树炭疽病菌对供试杀菌剂的敏感性,为生产上杨树炭疽病防治中杀菌剂的合理使用提供参考。
1 材料和方法 1.1 供试材料 1.1.1 菌株于2009年和2010年在北京市4个区县(石景山、昌平、密云和延庆)采集杨树炭疽病病叶,采用常规组织分离法[18]进行病原菌分离。取发病和健康交界处组织,用1.5%的次氯酸钠溶液表面消毒1~2 min,以无菌水冲洗3次,于无菌滤纸上吸干水分,将组织块置于常规PDA培养基上培养,并进行病原菌的分离和单孢纯化。根据培养性状、形态学特征以及多基因片段系统发育,就所分离获得的菌株进行鉴定[2],所有菌株(表 1)保存于北京林业大学森林培育和保护实验室。
|
|
表 1 供试杨树炭疽病菌 Table 1 Information of isolates of poplar anthracnose fungi in this study |
1.1.2 药剂
97%多菌灵(carbendazim)原药、95%苯醚甲环唑(difenoconazole)原药、97%戊唑醇(tebuconazole)原药及95%三唑酮(triadimefon)原药均由中国农业大学植物保护学院种子病理和药理学实验室提供;二甲基亚砜(DMSO),分析纯。将各药剂用DMSO配制为1 × 104 μg/mL的母液,于4 ℃保存,备用。
1.2 病原菌对杀菌剂的敏感性测定采用菌丝生长速率法[19]。用DMSO将供试药剂母液分别稀释为系列梯度浓度:多菌灵,0、0.03、0.05、0.075、0.1和0.15 μg/mL;苯醚甲环唑,0、0.05、0.1、0.5、2.5和5.0 μg/mL;戊唑醇,0、0.05、0.5、1.0、2.5和5.0 μg/mL;三唑酮,0、5.0、10、20、40和80 μg/mL。将药液按体积比1 : 1 000的比例分别加入到冷却至约50 ℃的PDA培养基中,充分混匀,制成系列浓度含药平板,以加入等体积DMSO的平板为对照。供试菌株在PDA培养基上、25 ℃黑暗培养4 d后,在菌落边缘同一圆周上打取直径为5 mm的菌饼,接种至含药PDA平板上,于25 ℃黑暗继续培养3 d后,采用十字交叉法测定各处理的菌落直径。每个浓度重复3次。按公式(1)求出各浓度药剂对菌丝生长的抑制率。
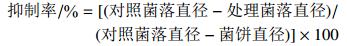
|
(1) |
以抑制率几率值为纵坐标(y),药剂浓度对数值为横坐标(x),求出毒力回归方程y=ax + b、相关系数(r)和药剂的有效抑制中浓度(EC50,μg/mL)。
1.3 数据统计分析采用DPS 7.05软件分析59株胶孢炭疽菌的敏感性频率分布,以EC50值为x轴,相应的频率(%)为y轴,绘制敏感性频率分布图。通过SPSS 18.0软件绘制不同树种来源胶孢炭疽菌的敏感性箱线图,并在α=0.05水平比较其差异显著性。将所得EC50值转换为lgEC50值,采用Spearman’s秩相关系数分析胶孢炭疽菌对不同杀菌剂之间敏感性的相关性[19]。
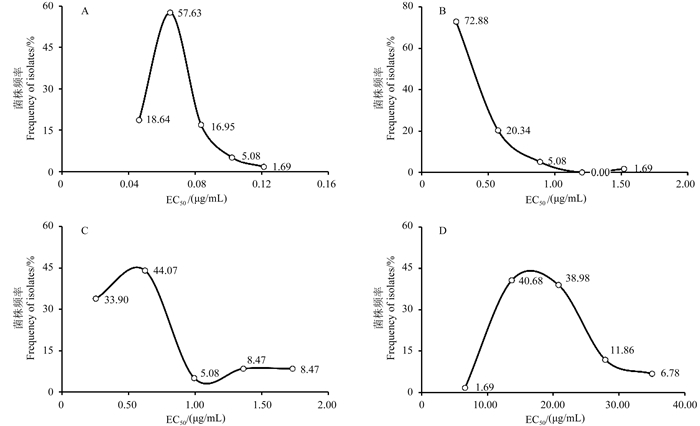
2 结果与分析 2.1 杨树炭疽病菌对4种杀菌剂的敏感性结果见图 1。供试63株杨树炭疽病菌株对多菌灵、苯醚甲环唑、戊唑醇和三唑酮的EC50值分别分布于0.037 1~0.130 1、0.102 5~4.279、0.069 1~9.160和3.053~50.54 μg/mL之间。其中,59株胶孢炭疽菌对多菌灵、苯醚甲环唑、戊唑醇和三唑酮的EC50值分别在0.037 1~0.130 1、0.102 5~1.680、0.069 1~1.917和3.053~38.59 μg/mL之间,最小EC50值和最大EC50值分别相差3.5、16.4、27.7和12.6倍,平均EC50值分别为(0.066 4±0.013 1)、(0.374 1±0.254 8)、(0.681 2±0.442 1)和(19.82±6.200) μg/mL。胶孢炭疽菌对多菌灵的敏感性频率分布呈连续单峰曲线(图 1A),可将该平均EC50值(0.066 4±0.013 1) μg/mL作为胶孢炭疽菌对多菌灵的敏感基线;而对苯醚甲环唑和戊唑醇的敏感性频率分布则均出现了不同程度的分化,呈双峰曲线(图 1B和图 1C),表明已出现敏感性下降的群体。
|
图 1 供试59株胶孢炭疽菌对多菌灵(A)、苯醚甲环唑(B)、戊唑醇(C)和三唑酮(D)的敏感性频率分布 Fig. 1 Frequency distribution of EC50 values of 59 C. gloeosporioides isolates to cabendazim (A), difenoconazole (B), tebuconazole (C) and triadimefon (D) |
供试4株炭疽菌对多菌灵、苯醚甲环唑、戊唑醇和三唑酮的最小EC50值和最大EC50值分别相差1.33、13.19、13.66和2.14倍(表 2),其中一株菌株Ca-4对苯醚甲环唑和戊唑醇的EC50值均大于4 μg/mL,可能对该两种药剂已出现敏感性下降的现象。
|
|
表 2 炭疽菌对4种供试杀菌剂的敏感性 Table 2 Sensitivities of C. aenigma isolates to four fungicides |
2.2 不同寄主来源胶孢炭疽菌对4种杀菌剂的敏感性
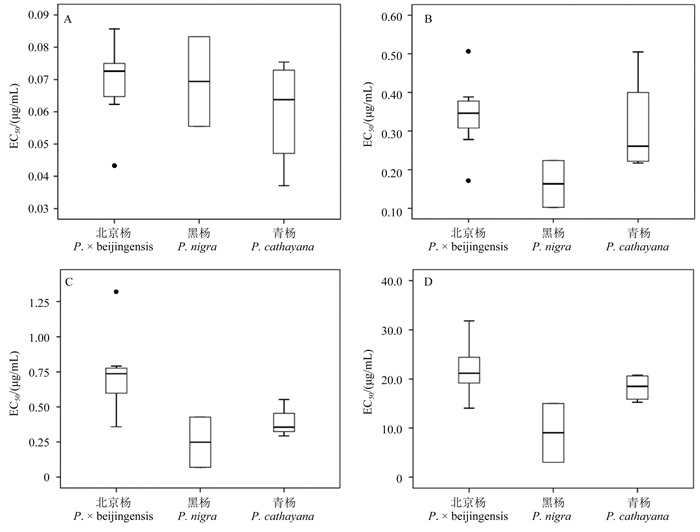
比较分别来自北京杨、青杨和黑杨的胶孢炭疽菌对供试药剂的敏感性,发现不同寄主来源胶孢炭疽菌对同种杀菌剂的敏感性之间没有显著性差异(P > 0.05),但仍存在一定的寄主差异,其中,对多菌灵的敏感性表现为黑杨 > 北京杨 > 青杨,对3种DMIs杀菌剂的敏感性趋势表现一致,均为北京杨 > 青杨 > 黑杨(图 2)。
|
注:圆点代表异常值。Note: Dots represent the extreme value. 图 2 不同寄主来源胶孢炭疽菌对多菌灵(A)、苯醚甲环唑(B)、戊唑醇(C)和三唑酮(D)的敏感性 Fig. 2 Sensitivities of C. gloeosporioides from different hosts to cabendazim (A), difenoconazole (B), tebuconazole (C) and triadimefon (D) |
2.3 胶孢炭疽菌对4种杀菌剂敏感性的相关性
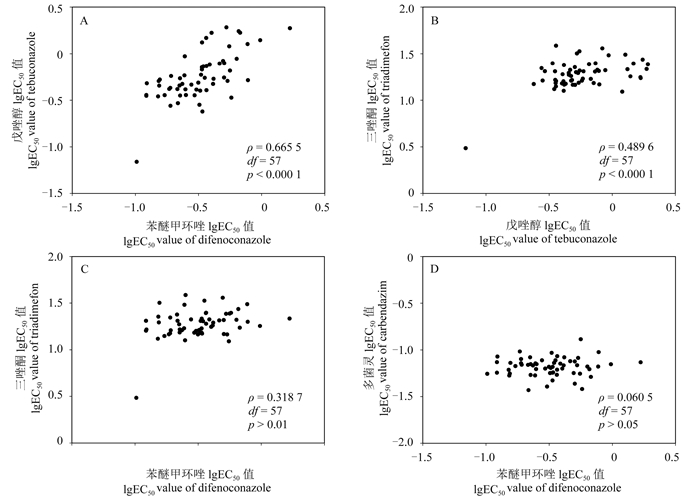
分析结果发现,59株胶孢炭疽菌对4种杀菌剂的敏感性表现出一定的相关性。其中,苯醚甲环唑和戊唑醇之间存在显著正相关性(ρ=0.665 5,P < 0.000 1)(图 3 A);戊唑醇与三唑酮之间存在一定的相关性(ρ=0.489 6,P < 0.000 1)(图 3B);但苯醚甲环唑与三唑酮和多菌灵之间均无显著相关性(P > 0.01)(图 3C和3D)。
|
注:A~D分别代表苯醚甲环唑与戊唑醇、戊唑醇与三唑酮、苯醚甲环唑与三唑酮及苯醚甲环唑与多菌灵的相关性分析。 Note:A. difenoconazole and tebuconazole; B. tebuconazole and triadimefon; C. difenoconazole and triadimefon; D. difenoconazole and carbendazim. 图 3 胶孢炭疽菌对4种杀菌剂敏感性的相关性分析 Fig. 3 Sensitivity correlation among the four fungicides |
3 结论与讨论
同一寄主上的炭疽病通常由多种炭疽病菌引发[3, 20-22],不同的炭疽病菌对同种杀菌剂的敏感性存在差异[17, 23],因此炭疽病防治过程中杀菌剂的选择应首先明确病原菌对药剂的敏感性。本研究测定了引起杨树炭疽病的两种炭疽病菌对4种杀菌剂的敏感性,结果表明,多菌灵对两种杨树炭疽病菌的抑制效果均最好,其次依次为苯醚甲环唑、戊唑醇和三唑酮。研究结果可为杨树炭疽病防治药剂的选择提供参考。
长期及不合理使用杀菌剂易导致病原菌产生抗药性[13]。以多菌灵为代表的苯并咪唑类杀菌剂主要作用于病原菌的β-微管蛋白[24],由于其作用位点单一,已有多种炭疽病菌对该类杀菌剂产生了抗药性[14-16, 25-26]。本研究中,2种杨树炭疽病菌对多菌灵表现敏感,且59株胶孢炭疽菌对多菌灵的敏感性频率分布呈连续单峰曲线,表明尚未出现敏感性下降的群体,因此可将其平均EC50值(0.066 4±0.013 1) μg/mL作为胶孢炭疽菌对多菌灵的敏感基线,为其田间抗药性菌株的检测与监测提供依据。
苯醚甲环唑、戊唑醇和三唑酮同属于SBIs杀菌剂中的C-14α-脱甲基酶抑制剂类(DMIs),能够抑制麦角甾醇生物合成途径中的关键性酶CYP51,破坏真菌细胞膜,导致细胞死亡[12]。因其优异的防治效果,已被广泛用于植物炭疽病的防治[10, 14, 27]。且由于植物病原真菌对该类杀菌剂的抗药性由多基因控制,抗性菌株的适合度较低,难以形成抗药性群体,因此DMIs杀菌剂被认为是低抗药性风险药剂[28-29]。然而,由于其作用位点单一且应用广泛,目前已有多种植物病原真菌对该类杀菌剂产生了抗药性[30-32],其中包括多种寄主来源的炭疽病菌[10, 15]。
本研究中,2种杨树炭疽病菌对DMIs杀菌剂也出现了敏感性下降的群体,其中,59株胶孢炭疽菌对苯醚甲环唑和戊唑醇的敏感性频率分布呈现双峰曲线,1株炭疽菌对苯醚甲环唑和戊唑醇的EC50值大于4 μg/mL,表明杨树炭疽病菌中可能已出现对DMIs杀菌剂的抗药性菌株。但由于本研究中仅分析了4株炭疽菌,菌株数量偏少,因此还需要进一步采集更多菌株进行分析确证。尽管对杨树炭疽病防治过程中的用药历史尚不清楚,但杨树炭疽病菌中对DMIs杀菌剂抗性菌株的出现仍值得注意。有报道称,不同寄主来源的炭疽病菌之间会发生交叉侵染[33-35],目前已明确杨树上的胶孢炭疽菌可侵染苹果及刺槐等[7],但尚不知对其他种类植物是否会发生交叉侵染。因此,后续研究还需采集更多杨树炭疽病菌样本,并通过遗传背景分析其与周边植物炭疽病菌的关系,明确其来源,以揭示杨树上炭疽病菌对DMIs杀菌剂敏感性下降的原因。
本研究还发现,杨树炭疽病菌对不同DMIs杀菌剂的敏感性之间存在一定的交互抗性关系,这与已有的报道[10, 36]一致。但其相关性也存在差异,其中苯醚甲环唑与戊唑醇存在显著正相关性,与三唑酮之间则无相关性;而戊唑醇与三唑酮之间存在较弱的正相关性。植物病原真菌对DMIs杀菌剂的抗药性涉及多种分子机制[24]。鉴于苯醚甲环唑、戊唑醇和三唑酮均作用于植物病原真菌的CYP51蛋白上[12],分析出现该现象的原因可能是由于苯醚甲环唑和戊唑醇在CYP51蛋白上具有相同的作用位点,而三唑酮则具有不同的作用位点。此外,同一靶标蛋白上不同氨基酸的突变也会不同程度地影响靶标蛋白与同种作用机制、不同化学结构杀菌剂之间的结合能力,从而导致植物病原真菌对不同药剂敏感性之间的相关程度不同[37]。本研究中杨树炭疽病菌对3种DMIs杀菌剂的敏感性差异可能与其抗性机制有关,具体的抗性分子机制尚待进一步研究。
综上所述,MBCs和DMIs杀菌剂是生产上常用于炭疽病防治的药剂,但长期及不合理的使用易导致炭疽病菌逐渐产生抗药性。虽然目前尚未检测到杨树炭疽病菌对多菌灵敏感性下降的群体,但其对DMIs杀菌剂已有敏感性下降的群体出现,因此,生产上应注意科学合理地应用这两类药剂防治杨树炭疽病,延缓或避免病原菌抗药性的产生。同时还应注意杨树炭疽病与其他植物炭疽病的交叉侵染特点,避免抗药性菌株在不同植物间的迁移。
| [1] |
贺伟, 杨旺, 沈瑞祥. 北京杨炭疽病的初步研究[J]. 森林病虫通讯, 1991 (4):7–9.
HE W, YANG W, SHEN R X. Preliminary study on anthracnose of Populus beijingensis[J]. Forest Pest Dis, 1991 (4):7–9. |
| [2] | LI Z, LIANG Y M, TIAN C M. Characterization of the causal agent of poplar anthracnose occurring in the Beijing region[J]. Mycotaxon, 2012, 120 (1):277–286. doi:10.5248/120.277 |
| [3] | LIU F, DAMM U, CAI L, et al. Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of proteaceae[J]. Fungal Divers, 2013, 61 (1):89–105. doi:10.1007/s13225-013-0249-2 |
| [4] |
武秀红. 伊犁地区杨树常见病害发生与防治[J]. 中国林业, 2012 (13):37.
WU X H. Occurrence and control of common diseases of poplar in Yili[J]. Forestry China, 2012 (13):37. |
| [5] |
李铮.北京地区杨树炭疽病病原鉴定及群体遗传分析[D].北京:北京林业大学, 2012:1.
LI Z. Identification, characteristics and population genetics analysis of pathogens causing poplar anthracnose in the Beijing region[D]. Beijing:Beijing Forestry University, 2012:1. http://cdmd.cnki.com.cn/article/cdmd-10022-1012350031.htm |
| [6] |
余露. 2009年北京主要林木有害生物发生趋势[J]. 农药市场信息, 2009 (4):43.
YU L. Occurrence trend of Beijing major seedling pests in 2009[J]. Pestic Market News, 2009 (4):43. |
| [7] |
朱克恭. 树木炭疽病[J]. 森林病虫通讯, 1989 (2):37–40.
ZHU K G. Tree anthracnose[J]. Forest Pest Dis, 1989 (2):37–40. |
| [8] |
沈瑞祥. 杨树病害综述[J]. 世界林业研究, 1989 (2):39–43.
SHEN R X. A review on poplar diseases[J]. World Forestry Res, 1989 (2):39–43. |
| [9] | MACKENZIE S J, MERTELY J C, PERES N A. Curative and protectant activity of fungicides for control of crown rot of strawberry caused by Colletotrichum gloeosporioides[J]. Plant Dis, 2009, 93 (8):815–820. doi:10.1094/PDIS-93-8-0815 |
| [10] |
邓维萍, 杨敏, 杜飞, 等. 葡萄胶孢炭疽菌对3种麦角甾醇脱甲基抑制剂类杀菌剂的敏感性[J]. 农药学学报, 2011,13 (3):245–252.
DENG W P, YANG M, DU F, et al. Sensitivity of Colletotrichum gloeosporioides causing grape anthracnose to three sterol demethylation inhibitor (DMI) fungicides[J]. Chin J Pestic Sci, 2011, 13 (3):245–252. |
| [11] | WONG F P, MIDLAND S L. Sensitivity distributions of California populations of Colletotrichum cereale to the DMI fungicides propiconazole, myclobutanil, tebuconazole, and triadimefon[J]. Plant Dis, 2007, 91 (12):1547–1555. doi:10.1094/PDIS-91-12-1547 |
| [12] |
叶滔, 马志强, 毕秋艳, 等. 植物病原真菌对甾醇生物合成抑制剂类(SBIs)杀菌剂的抗药性研究进展[J]. 农药学学报, 2012,14 (1):1–16.
YE T, MA Z Q, BI Q Y, et al. Research advances on the resistance of plant pathogenic fungi to SBIs fungicides[J]. Chin J Pestic Sci, 2012, 14 (1):1–16. |
| [13] | RUSSELL P E. Fungicide resistance:occurrence and management[J]. J Agric Sci, 1995, 124 (3):317–323. doi:10.1017/S0021859600073275 |
| [14] | XU X F, LIN T, YUAN S K, et al. Characterization of baseline sensitivity and resistance risk of Colletotrichum gloeosporioides complex isolates from strawberry and grape to two demethylation-inhibitor fungicides, prochloraz and tebuconazole[J]. Australas Plant Pathol, 2014, 43 (6):605–613. doi:10.1007/s13313-014-0321-8 |
| [15] |
陈聃, 时浩杰, 吴慧明, 等. 浙江省葡萄炭疽菌对甲基硫菌灵和戊唑醇的抗药性研究[J]. 果树学报, 2013,30 (4):665–668.
CHEN D, SHI H J, WU H M, et al. Resistance of Colletotrichum gloeosporioides causing grape ripe rot to thiophanate-methyl and tebuconazole in Zhejiang[J]. J Fruit Sci, 2013, 30 (4):665–668. |
| [16] | WONG F P, DE LA CERDA K A, HERNANDEZ-MARTINEZ R, et al. Detection and characterization of benzimidazole resistance in California populations of Colletotrichum cereale[J]. Plant Dis, 2008, 92 (2):239–246. doi:10.1094/PDIS-92-2-0239 |
| [17] | PERES N A R, SOUZA N L, PEEVER T L, et al. Benomyl sensitivity of isolates of Colletotrichum acutatum and C. gloeosporioides from citrus[J]. Plant Dis, 2004, 88 (2):125–130. doi:10.1094/PDIS.2004.88.2.125 |
| [18] |
方中达.
植病研究方法[M].3版. 北京: 中国农业出版社, 1998 : 124 .
FANG Z D. Methodology of plant pathology[M].3rd ed. Beijing: China Agriculture Press, 1998 : 124 . |
| [19] | CHEN L, ZHU S S, LU X H, et al. Assessing the risk that Phytophthora melonis can develop a point mutation (V1109L) in CesA3 conferring resistance to carboxylic acid amide fungicides[J]. PLoS One, 2012, 7 (7):3597–3610. |
| [20] | THAN P P, JEEWON R, HYDE K D, et al. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand[J]. Plant Pathol, 2008, 57 (3):562–572. doi:10.1111/j.1365-3059.2007.01782.x |
| [21] | FREEMAN S, KATAN T, SHABI E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits[J]. Plant Dis, 1998, 82 (6):596–605. doi:10.1094/PDIS.1998.82.6.596 |
| [22] | LIMA N B, BATISTA M V D A, DE MORAIS JR M A, et al. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil[J]. Fungal Divers, 2013, 61 (1):75–88. doi:10.1007/s13225-013-0237-6 |
| [23] | ADASKAVEG J E, HARTIN R J. Characterization of Colletotrichum acutatum isolates causing anthracnose of almond and peach in California[J]. Phytopathology, 1997, 87 (9):979–987. doi:10.1094/PHYTO.1997.87.9.979 |
| [24] | MA Z H, MICHAILIDES T J. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi[J]. Crop Prot, 2005, 24 (10):853–863. doi:10.1016/j.cropro.2005.01.011 |
| [25] |
詹儒林, 李伟, 郑服丛. 芒果炭疽病菌对多菌灵的抗药性[J]. 植物保护学报, 2005,32 (1):71–76.
ZHAN R L, LI W, ZHENG F C. Studies on carbendazim-resistance of Colletotrichum gloeosporioides on mango fruit[J]. Acta Phytophylacica Sinica, 2005, 32 (1):71–76. |
| [26] |
韩国兴, 礼茜, 孙飞洲, 等. 杭州地区草莓炭疽病病原鉴定及其对多菌灵和乙霉威的抗药性[J]. 浙江农业科学, 2009 (6):1169–1172.
HAN G X, LI Q, SUN F Z, et al. Etiology of anthrancnose of strawberry in Hangzhou and its resistance for carbendazim and diethofencarb[J]. J Zhejiang Agric Sci, 2009 (6):1169–1172. |
| [27] |
刘霞, 杨克强, 朱玉凤, 等. 8种杀菌剂对核桃炭疽病病原菌胶孢炭疽菌的室内毒力[J]. 农药学学报, 2013,15 (4):412–420.
LI X, YANG K Q, ZHU Y F, et al. Laboratory toxicity of eight fungicides against Colletotrichum gloeosporioides causing walnut anthracnose[J]. Chin J Pestic Sci, 2013, 15 (4):412–420. |
| [28] | FUCHS A, DRANDAREVSKI C A. The likelihood of development of resistance to systemic fungicides which inhibit ergosterol biosynthesis[J]. Netherlands J Plant Pathol, 1976, 82 (2):85–87. doi:10.1007/BF01976954 |
| [29] | KÖLLER W, SCHEINPFLUG H. Fungal resistance to sterol biosythesis inhibitors:a new challenge[J]. Plant Dis, 1987, 71 (12):1066–1074. doi:10.1094/PD-71-1066 |
| [30] | BLATTER R H E, BROWN J K M, WOLFE M S. Genetic control of the resistance of Erysiphe graminis f. sp. hordei to five triazole fungicides[J]. Plant Pathol, 1998, 47 (5):570–579. doi:10.1046/j.1365-3059.1998.00276.x |
| [31] | KARAOGLANIDIS G S, THANASSOULOPOULOS C C, IOANNIDIS P M. Fitness of Cercospora beticola field isolates-resistant and -sensitive to demethylation inhibitor fungicides[J]. Eur J Plant Pathol, 2001, 107 (3):337–347. doi:10.1023/A:1011219514343 |
| [32] | STERGIOPOULOS I, VAN NISTELROOY J G M, KEMA G H J, et al. Multiple mechanisms account for variation in base-line sensitivity to azole fungicides in field isolates of Mycosphaerella graminicola[J]. Pest Manag Sci, 2003, 59 (12):1333–1343. doi:10.1002/(ISSN)1526-4998 |
| [33] | FREEMAN S, SHABI E. Cross-infection of subtropical and temperate fruits by Colletotrichum species from various hosts[J]. Physiol Mol Plant Pathol, 1996, 49 (6):395–404. doi:10.1006/pmpp.1996.0062 |
| [34] | HARP T, KUHN P, ROBERTS P D, et al. Management and cross-infectivity potential of Colletotrichum acutatum causing anthracnose on bell pepper in Florida[J]. Phytoparasitica, 2014, 42 (1):31–39. doi:10.1007/s12600-013-0334-9 |
| [35] | KIM H R, LIM T H, KIM Y H, et al. Potential of cross-infection of Colletotrichum species causing anthracnose in persimmon and pepper[J]. Plant Pathol J, 2009, 25 (1):13–20. doi:10.5423/PPJ.2009.25.1.013 |
| [36] | KÖLLER W, WILCOX W F, BARNARD J, et al. Detection and quantification of resistance of Venturia inaequalis populations to sterol demethylation inhibitors[J]. Phytopathology, 1997, 87 (2):184–190. doi:10.1094/PHYTO.1997.87.2.184 |
| [37] | ZHOU Y X, CHEN L, HU J, et al. Resistance mechanisms and molecular docking studies of four novel QoI fungicides in Peronophythora litchii[J]. Sci Rep, 2015, 5 :17466. doi:10.1038/srep17466 |
 2016, Vol. 18
2016, Vol. 18


