姜黄素(curcumin),化学名称为[1,7-二(4-羟基-3-甲氧基苯基)-1,6-庚二烯-3,5-二酮],结构式见(Ⅰ),是一种植物源活性物质,主要提取于中药植物姜黄Curcuma long L.中,可作为食品添加剂[1],同时具有多种药理活性[2, 3]及杀虫和杀菌活性等[4, 5]。
张永强等[6]研究表明,姜黄素对朱砂叶螨Tetranychus cinnabarinus Boisduval雌成螨和若螨具有显著的触杀活性,48 h的LC50值分别为2.64和3.27 mg/mL,对成螨产卵也存在抑制作用。罗金香等[7]报道,用姜黄素的衍生物双去甲氧基姜黄素(bisdemethoxycurcumin)处理朱砂叶螨后,其乙酰胆碱酯酶(AchE)活性下降了16.77%,Ca2+-ATP酶活性下降17.55%,而谷胱甘肽S-转移酶(GSTs)和羧酸酯酶(CarE)活性均上升,该结果初步表明,双去甲氧基姜黄素对螨的致死作用可能与神经系统相关酶有关,但具体的作用靶标尚未明确。
朱砂叶螨T.cinnabarinus是一种重要的世界性经济害螨,危害多种作物[8, 9, 10],具有成熟期短、繁殖率高和活动范围小等特点,目前已对多种化学杀螨剂产生了严重的抗药性[11]。因此,迫切需要寻找新的螨类控制剂。本研究室在研究过程中发现,用姜黄素处理朱砂叶螨后,会导致螨大量失水,体表皱缩,而这些症状与螨体壁结构密切相关。几丁质作为螨体壁角质层的主要组成成分,其合成与降解对螨的生长发育至关重要[12, 13]。然而目前尚未见有关姜黄素对螨类几丁质的合成及降解酶系影响的研究报道。笔者以几丁质酶为切入点,研究了姜黄素对朱砂叶螨几丁质降解的影响,以期探明姜黄素对朱砂叶螨的作用机制。
1 材料与方法 1.1 供试材料 1.1.1 试剂姜黄素(curcumin),分析纯,购自河北食品添加剂有限公司;对照药剂98%螺螨酯(spirodiclofen)原药,购自海利尔药业集团股份有限公司;Total RNAprep Pure Tissue Kit试剂盒和DNA Purification Kit试剂盒购自天根生化科技(北京)有限公司;PrimeScriptTM Ⅱ 1st Strand cDNA Synthesis Kit试剂盒、LA Taq酶、pMD-19T easy vector试剂盒、DH5α和SYBR®Premix Ex Taq TM Ⅱ,均购自TAKARA公司。
1.1.2 供试螨类朱砂叶螨T.cinnabarinus,采自重庆市北碚区田间,在人工气候室内(25 ℃±1 ℃,相对湿度75%~80%,L : D=14 h :10 h)用盆栽豇豆Vigna unguiculata饲养多年,期间未施用任何药剂。
1.1.2.1 不同发育阶段螨的收集在直径7.0 cm的培养皿内,依次铺上脱脂棉、滤纸和直径5.0 cm的豇豆苗叶片,每个叶片上放置大约30头雌成螨,待其产卵24 h后移除这些雌成螨,将所产的卵置于人工气候室内(25 ℃±1 ℃,相对湿度75%~80%,L : D=14 h : 10 h)培养。从卵开始,依次收集幼螨、若螨和3~5日龄成螨[14],于-80 ℃下储存,用于总RNA提取。
1.1.2.2 不同浓度姜黄素处理后螨的收集称取适量姜黄素,先用少量丙酮溶解后,再用含0.1%吐温-80的水溶液稀释成4~0.25 mg/mL的5个浓度梯度供试。以含有0.1%吐温-80的ddH2O为对照(CK1),以用ddH2O稀释5 000倍的螺螨酯为药剂对照(CK2)。当螨卵发育至若螨时,采用叶碟浸渍法[14]将叶片在各浓度药剂中浸渍5 s,吸干螨周围的液体,置于人工气候室内(条件同1.1.2.1节)24 h收集存活若螨,用于总RNA提取。
1.2 试验方法 1.2.1 毒力测定参照联合国粮农组织(FAO)推荐的玻片浸渍法[15]测定。
1.2.2 朱砂叶螨几丁质酶基因的克隆及序列分析朱砂叶螨总RNA使用RNAprep Pure Tissue Kit试剂盒提取,第一链cDNA使用PrimeScriptTM Ⅱ 1st Strand cDNA Synthesis Kit试剂盒合成,具体步骤均参照试剂盒说明书进行。参考朱砂叶螨近缘种二斑叶螨Tetranychus urticae的全基因组序列(http://bioinformatics.psb.ugent.be/orcae/overview/Teur),设计出用于扩增朱砂叶螨几丁质酶基因(TcCHTs)的引物(表 1)。PCR反应体系和反应程序参照文献[16]进行。PCR反应产物在1%的琼脂糖凝胶中电泳,按照DNA Purification Kit说明书将目的片段切胶纯化。将纯化后的目的片段与载体pMD-19T easy vector过夜连接,连接产物转化至感受态细胞DH5α中,将重组质粒的菌液进行PCR扩增鉴定,并将鉴定出的阳性菌液送至上海Invitrogen公司测序。测序结果利用DNAMAN、BioXM2.6和Clustal X等软件[17]进行分析,采用MEGA5.10软件[18]的邻接法(neighbor-joining)构建系统发育树,各分支均进行1 000次重复检验。
|
| 表 1 朱砂叶螨TcCHTs基因特异性扩增引物列表 Table 1 Primers for cloning the sequences of CHT genes in Tetranychus cinnabarinus |
根据1.2.2几丁质酶cDNA克隆结果,使用Primer 3.0设计用于定量的引物,见表 2,选取RPS18为内参基因[19]。定量PCR反应体系和反应程序参照文献[16]进行,相对定量表达数据采用2−ΔΔCt方法分析[20]。
|
| 表 2 朱砂叶螨TcCHTs基因RT-qPCR引物列表 Table 2 Primers for Real-time RCR of CHT genes in Tetranychus cinnabarinus |
姜黄素和对照药剂螺螨酯对朱砂叶螨雌成螨48 h的LC50值分别为2.424和0.696 mg/mL(表 3),尽管姜黄素的毒力低于螺螨酯的,但作为天然活性物质,姜黄素已具有很好的杀螨活性。
|
| 表 3 姜黄素及螺螨酯对朱砂叶螨雌成螨的毒力测定结果(48 h) Table 3 Toxicity of acaricide against Tetranychus cinnabarinus female adults (48 h) |
将6条cDNA(TcCHIT1、TcCHIT2、TcCHIT3、TcCHT4、TcCHT5和TcCHT6)序列克隆结果提交至GenBank(登录号分别为:KT956964、KT956965、KT956966、KT956967、KT956968和KT956969),得到其碱基序列、氨基酸序列及预测的结构域见图 1。由TcCHIT1、TcCHIT2、TcCHIT3和TcCHT4推导的氨基酸序列有一个信号肽、一个催化结构域和一个几丁质结构域,而TcCHT5和TcCHT6没有信号肽,存在一个跨膜域和一个催化结构域。预测蛋白质的分子质量分别为60.80、69.03、104.32、59.75、45.63和49.05 kDa,等电点分别为5.44、8.65、6.23、6.07、6.12和8.27(表 4)。将这6条基因碱基序列与二斑叶螨基因组数据库中进行BLASTP比对,每条基因与相对应的二斑叶螨的基因相似性均为98%~99%。构建的系统发育树结果(图 2)显示,6条基因分在不同的分支上,但因具有高度的相似性,故与各自对应的二斑叶螨基因聚在同一分支上。
 |
注:单线表示信号肽,双线表示糖基化位点,灰色标注表示催化结构域,黑色背景表示几丁质结合域,绿色背景表示跨膜结构域。 Note: Signal peptides were underlined. The N-glycosylation sites were indicated with double lines. The catalytic domains were shaded. The chitin-binding domains were in white with a black background. The transmembrane helice were in white with a green background. A: TcCHIT1; B: TcCHIT2; C: TcCHIT3; D: TcCHT4; E: TcCHT5; F: TcCHT6 图 1 朱砂叶螨 TcCHTs 基因 cDNA 序列及推导的氨基酸序列 Fig. 1 Nucleotide and deduced amino acid of TcCHTs cDNA sequence |
|
| 表 4 朱砂叶螨TcCHTs基因cDNA序列信息 Table 4 The complete sequence information of CHT genes of Tetranychus cinnabarinus |
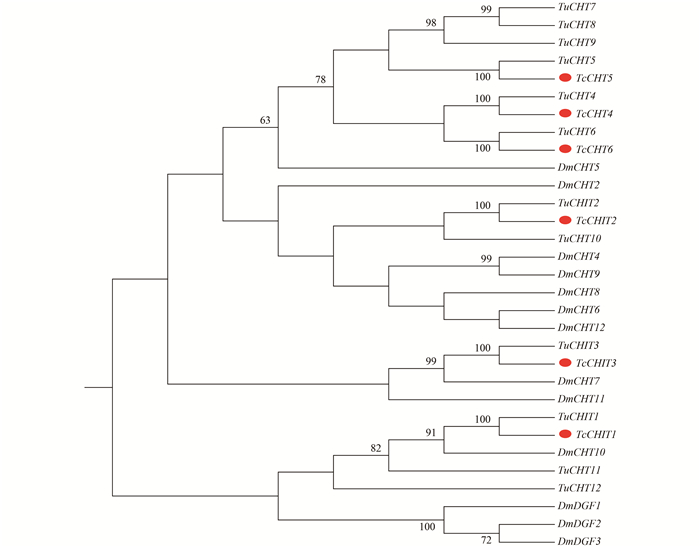 |
注:采用MEGA 5.10的邻接法构建发育树,各分支进行1 000检验。所需物种CHT 序列从二斑叶螨全基因库和NCBI获得。 Note: A phylogenetic tree was constructed by MEGA 5.10 based on the neighbor-joining method according to amino acid sequences. Bootstrap support values with 1 000 were shown on the branches. Reference sequences were obtained from the genome database of Tetranychus urticae and the NCBI database. Tu: 二斑叶螨 Tetranychus urticae (TuCHIT1: tetur01g11910; TuCHIT2: tetur02g08940; TuCHIT3: tetur03g04960; TuCHT4: tetur17g02930; TuCHT5: tetur14g03830; TuCHT6: tetur14g03950). Dm: 黑腹果蝇 Drosophila melanogaster (DmCHT2: NP_477298.2; DmCHT4: NP_524962.2; DmCHT5: NP_650314.1; DmCHT6: NP_572598.2; DmCHT7: NP_647768.3; DmCHT8: NP_611542.2; DmCHT9: NP_611543.3; DmCHT10: NP_001036422.1; DmCHT11: NP_572361.1; DmCHT12: NP_726022.1; DmDGF1: AAC99417.1; DmDGF2: AAC99418.1; DmDGF3: AAC99419.1) 图 2 不同物种CHT基因进化树分析 Fig. 2 Phylogenetic analysis among TcCHTs, TuCHTs, and DmCHTs |
采用RT-qPCR测定6条基因TcCHTs在朱砂叶螨4个不同发育阶段的表达量,结果见图 3。根据克隆序列的分析结果,6条基因有不同的结构,不同阶段螨的基因表达也存在差异。总体而言,幼螨和若螨阶段的基因表达量显著高于卵和成螨阶段,其中TcCHIT1、TcCHIT2、TcCHIT3和TcCHT4 4条基因这种差异更加显著,前者的表达量高出后者近百倍。
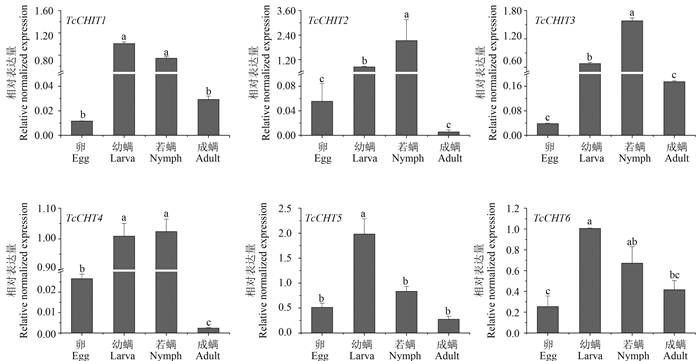 |
注:每个发育阶段柱上不同的字母表示具有显著差异(SPSS 17.0, ANOVA , Duncan P < 0.05),RPS18为内参基因。 Note: Different letters on the error bars showed significant differences by Duncan (P < 0.05). RPS18 was used as the reference gene. 图 3 朱砂叶螨不同发育阶段TcCHTs的表达模式 Fig. 3 Expression levels of TcCHTs across different developmental stages of Tetranychus cinnabarinus |
用不同浓度的姜黄素处理朱砂叶螨若螨24 h后测定若螨中几丁质酶基因的表达量,结果见图 4。与对照CK1(含0.1%吐温-80的ddH2O)相比,6条几丁质酶基因的表达量普遍降低,但降低程度有所不同。TcCHIT1和TcCHT5两条基因的下降更为显著且下降趋势平缓。用对照药剂螺螨酯(CK2)处理后,与CK1相比,TcCHITs的表达量升高。
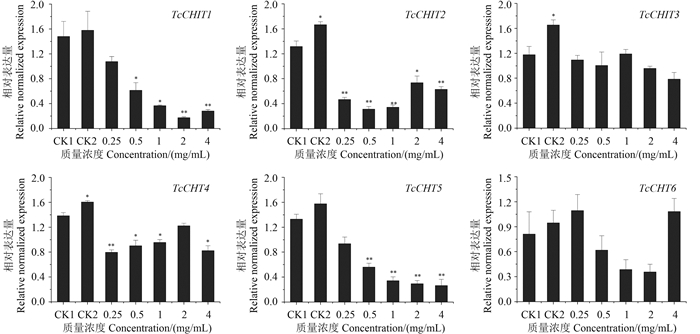 |
注:以含有0.1%吐温-80的ddH2O为对照(CK1),稀释5 000倍的螺螨酯为药剂对照(CK2)。*和**分别表示与对照CK1相比分别存在显著和极显著差异,* (P < 0.05), ** (P < 0.01, t-test);RPS18 为内参基因。 Note: Water containing 0.1% Tween-80, was used for treatment of T. cinnabarinus as the control (CK1), and spirodiclofen (diluted 5 000 times) was used as the positive control (CK2). *and ** on the error bar indicated a significant difference between treatment and control group (CK1), * (P < 0.05) or ** (P < 0.01, t-test). RPS18 was used as the reference gene. 图 4 不同浓度姜黄素对朱砂叶螨TcCHTs mRNA表达水平的影响(24 h) Fig. 4 Effect of curcumin on the mRNA expression of TcCHTs (24 h) |
昆虫表皮几丁质的降解主要是由几丁质酶和β-N-乙酰葡糖胺糖苷酶共同完成[12],几丁质酶可将几丁质内切为几丁寡糖,对于几丁质的降解有至关重要的作用。相对于植物和细菌而言,昆虫和螨类几丁质酶相关分子生物学的研究起步较晚。1993年,第一条昆虫几丁质酶基因的cDNA序列从烟草天蛾Manduca sexta中被分离鉴定出来[21],此后,多种昆虫的几丁质酶基因序列得以克隆鉴定[22, 23, 24, 25, 26]。昆虫几丁质酶具有不同的结构部位和功能区域,不同昆虫间的几丁质酶差异也较大,因而对昆虫几丁质酶进行分类存在诸多困难。经过长期研究,最终将昆虫几丁质酶分为了8组[13]。本研究克隆并分析了朱砂叶螨6条几丁质酶基因序列,明确了这6条基因在螨不同发育阶段的表达差异,进一步丰富了螨类相关几丁质酶的分子研究。同时明确了姜黄素对朱砂叶螨几丁质酶基因表达的抑制作用,初步明确了姜黄素杀螨活性可能与几丁质降解过程有关。
在朱砂叶螨4个不同发育阶段中,6条几丁质酶基因在幼螨和若螨的表达量显著高于卵和成螨阶段,这与Yang等[26]和Zhu等[27]的研究结果一致。各条基因在螨不同阶段的表达量也有较大差别,其中TcCHIT1、 TcCHIT2、TcCHIT3和TcCHT4 4条基因的表达量在幼螨和若螨时期高出卵和成螨时期近百倍,而TcCHT5和TcCHT6仅仅高出约5倍,这可能与这6条几丁质酶基因的结构部位和功能结构有关。由几丁质酶基因序列分析结果可以看出:前4条基因编码的氨基酸具有信号肽,可能是分泌型蛋白,后2条基因无信号肽,存在跨膜结构域,很可能是膜蛋白,不同的功能可能导致基因表达量存在差异。
用姜黄素处理朱砂叶螨若螨后,6条几丁质酶基因表达量均呈下降趋势,进而可能引起酶活性降低。由于目前尚未见关于朱砂叶螨几丁质酶6条基因的相关功能研究报道,笔者借鉴昆虫几丁质酶基因的分类[28]——黑腹果蝇D.melanogaster几丁质酶基因5(DmCHT5)和几丁质酶基因10(DmCHT10)分别属于几丁质酶Ⅰ组和Ⅱ组,而Ⅰ组和Ⅱ组与昆虫的蜕皮息息相关[29]。根据本研究系统发育树的构建结果,TcCHIT1以91%的置信度与DmCHT10聚在同一分支上,初步说明TcCHIT1可能属于几丁质酶Ⅱ组,即直接影响螨的蜕皮过程。由姜黄素处理朱砂叶螨24 h后,TcCHIT1的表达量随姜黄素质量浓度的增大呈显著下降趋势的结果看,姜黄素对螨表皮蜕皮有抑制作用,通过影响螨表皮几丁质的降解而导致螨死亡。对照药剂螺螨酯主要通过抑制脂肪的合成,破坏能量代谢最终杀死害螨[30],有关其对几丁质酶的作用少有报道。本研究结果表明,用螺螨酯处理朱砂叶螨后,可引起几丁质酶基因表达量升高。笔者在研究姜黄素和螺螨酯对几丁质酶基因表达的影响之前,曾对其是否影响朱砂叶螨的几丁质酶活性进行了测定,发现经姜黄素和螺螨酯处理后,其几丁质酶活性均下降(结果待发表)。推测可能是由于螺螨酯的作用靶标并非几丁质酶,当几丁质酶基因表达量升高时出现应激反应,致使其酶活性反而降低。姜黄素处理后几丁质酶基因表达量和酶活性均降低,说明其对几丁质酶的抑制作用是相对长久的。
目前有关姜黄素对朱砂叶螨的作用机理研究相对缺乏,本研究从朱砂叶螨几丁质酶降解几丁质方面,分析了姜黄素对朱砂叶螨几丁质酶的影响,推测其抑制了螨的蜕皮发育过程,从而影响螨的寿命。由于植物源杀螨活性物质往往具有多作用位点[31],有关姜黄素杀螨作用的具体作用靶标有待进一步研究。
| [1] | ZSILA F, BIKÁDI Z, SIMONYI M. Molecular basis of the cotton effects induced by the binding of curcumin to human serum albumin[J/OL]. Tetrahedron:Asymmetry, 2003, 14(16):2433-2444[2015-08-02]. http://www.sciencedirect.com/science/article/pii/S0957416603004865. doi:10.1016/S0957-4166(03) 00486-5. |
| [2] | MARCHIANI A, ROZZO C, FADDA A, et al. Curcumin and curcumin-like molecules:from spice to drugs[J/OL]. Curr Med Chem, 2014, 21(2):204-222[2015-08-02].http://www. ingentaconnect.com/content/ben/cmc/2014/00000021/00000002/art00004. |
| [3] | ZHANG D W, FU M, GAO S H, et al. Curcumin and diabetes:a systematic review[J/OL]. Evid-Based Compl Alt Med, 2013, 2013:636053[2015-08-02].http://www.hindawi.com/journals/ecam/2013/636053/. doi:10.1155/2013/636053.. |
| [4] | TRIPATHI A K, PRAJAPATI V, VERMA N, et al. Bioactivities of the leaf essential oil of Curcuma longa(var. ch-66) on three species of stored-product beetles(Coleoptera)[J/OL]. J Econ Entomol, 2002, 95(1):183-189[2015-08-02].http://jee.oxford-journals.org/content/95/1/183.abstract. doi:10.1603/0022-0493-95.1.183. |
| [5] | ARAÚJO C A C, LEON L L. Biological activities of Curcuma longa L.[J/OL]. Mem Inst Oswaldo Cruz, 2001, 96(5):723-728[2015-08-02].http://www.scielo.br/scielo.php?pid=S0074-02762001000500026&script=sci_arttext. doi:10.1590/S0074-02762001000500026. |
| [6] | 张永强, 丁伟, 赵志模. 姜黄素类化合物对朱砂叶螨的生物活性[J]. 昆虫学报, 2007, 50(12):1304-1308. ZHANG Y Q, DING W, ZHAO Z M. Biological activities of curcuminoids against Tetranychus cinnabarinus Boisduval(Acari:Tetranychidae)[J]. Acta Entomologica Sinica, 2007, 50(12):1304-1308. |
| [7] | 罗金香, 丁伟, 张永强, 等. 双去甲氧基姜黄素及其N-甲基吡唑衍生物对朱砂叶螨生物活性和多种酶活性的影响[J]. 中国农业科学, 2013, 46(13):2833-2844.LUO J X, DING W, ZHANG Y Q, et al. Acaricidal activity of Bisdemethoxycurcumin and N-methylpyrazolebisde-methoxycurcumin against Tetranychus cinnabarinus(Boisduval) and their effects on enzymes activity in the mite[J]. Scientia Agricultura Sinica, 2013, 46(13):2833-2844. |
| [8] | HAZAN A, GERSON U, TAHORI A S. Spider mite webbing. Ⅰ. The production of webbing under various environmental conditions[J]. Acarologia, 1974, 16(1):68-84. |
| [9] | HO C C, LO K C, CHEN W H. Spider mites(Acari:Tetranychidae) on various crops in Taiwan[J]. J Agric Res China, 1997, 46(4):333-346. |
| [10] | ÇAKMAK I, BAŞPINAR H, MADANLAR N. Control of the carmine spider mite Tetranychus cinnabarinus boisduval by the predatory mite Phytoseiulus persimilis(athias-henriot) in protected strawberries in Aydin, Turkey[J/OL]. Turk J Agric For, 2005, 29(4):259-265[2015-08-03].http://journals.tubitak.gov.tr/agriculture/issues/tar-05-29-4/tar-29-4-5-0405-8.pdf. |
| [11] | HE L, ZHAO Z M, DENG X P, et al. Resistance risk assessment:realized heritability of resistance to methrin, abamectin, pyridaben and their mixtures in the spider mite, Tetranychus cinnabarinus[J/OL]. Int J Pest Manag, 2003, 49(4):271-274[2015-08-03].http://www.tandfonline.com/doi/abs/10.1080/0967087031000101043. |
| [12] | MERZENDORFER H, ZIMOCH L. Chitin metabolism in insects:structure, function and regulation of chitin synthases and chitinases[J/OL]. J Exp Biol, 2003, 206(24):4393-4412[2015-08-04].http://jeb.biologists.org/content/206/24/4393.short. doi:10.1242/jeb.00709. |
| [13] | ARAKANE Y, MUTHUKRISHNAN S. Insect chitinase and chitinase-like proteins[J/OL]. Cell Mol Life Sci, 2010, 67(2):201-216[2015-08-04].http://link.springer.com/article/10.1007/s00018-009-0161-9. |
| [14] | MICHEL A P, MIAN M A, DAVILA-OLIVAS N H, et al. Detached leaf and whole plant assays for soybean aphid resistance:differential responses among resistance sources and biotypes[J/OL]. J Econ Entomol, 2010, 103(3):949-957[2015-08-06].http://jee.oxfordjournals.org/content/jee/103/3/949.full.pdf. doi:10.1603/EC09337. |
| [15] | BUSVINE J R. Recommended methods for measurement of pest resistance to pesticide[R]. FAO Plant Production and Protection Paper, 1980:49-54. |
| [16] | LUO Y J, YANG Z G, XIE D Y, et al. Molecular cloning and expression of glutathione S-transferases involved in propargite resistance of the carmine spider mite, Tetranychus cinnabarinus(Boisduval)[J/OL]. Pestic Biochem Phys, 2014, 114:45-51[2015-08-07]. http://www.sciencedirect.com/science/article/pii/S004835751400128X. doi:10.1016/j.pestbp.2014.07.004. |
| [17] | XIA W K, DING T B, NIU J Z, et al. Exposure to diflubenzuron results in an up-regulation of a chitin synthase 1 gene in citrus red mite, Panonychus citri (Acari:Tetranychidae)[J/OL]. Int J Mol Sci, 2015, 15(3):3711-3728[2015-08-07].http://www.mdpi.com/1422-0067/15/3/3711/htm. doi:10.3390/ijms15033711. |
| [18] | TAMURA K, PETERSON D, PETERSON N, et al. MEGA5:molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods[J/OL]. Mol Biol Evol, 2011, 28(10):2731-2739[2015-08-07].http://mbe.oxfordjournals.org/content/28/10/2731.short. doi:10.1093/molbev/msr121. |
| [19] | SUN W, JIN Y, HE L, et al. Suitable reference gene selection for different strains and developmental stages of the carmine spider mite, Tetranychus cinnabarinus, using quantitative real-time PCR[J/OL]. J Insect Sci, 2010, 10:208[2015-08-07]. http://jinsect-science.oxfordjournals.org/content/10/1/208.abstract. doi:10.1673/031.010.20801. |
| [20] | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method[J/OL]. Methods, 2001, 25(4):402-408[2015-08-07]. http://www.sciencedirect.com/science/article/pii/S1046202301912629. doi:10.1006/meth.2001.1262. |
| [21] | KRAMER K J, CORPUZ L, CHOI H K, et al. Sequence of a cDNA and expression of the gene encoding epidermal and gut chitinases of Manduca sexta[J/OL]. Insect Biochem Molec Biol, 1993, 23(6):691-701[2015-08-09]. http://www.sciencedirect.com/science/article/pii/096517489390043R. doi:10.1016/0965-1748(93)90043-R. |
| [22] | DE LA VEGA H, SPECHT C A, LIU Y, et al. Chitinases are a multi-gene family in Aedes, Anopheles and Drosophila [J/OL]. Insect Mol Biol, 1998, 7(3):233-239[2015-08-09].http://europepmc.org/abstract/med/9662472. doi:10.1111/j.1365-2583. 998.00065.x. |
| [23] | FEIX M, GLÖEGGLER S, LONDERSHAUSEN M, et al. A cDNA encoding a chitinase from the epithelial cell line of Chironomus tentans (Insecta, Diptera) and its functional expression[J/OL]. Arch Insect Biochem Physiol, 2000, 45(1):24-36[2015-08-09].http://onlinelibrary.wiley.com/doi/10.1002/1520-6327(200009)45:1<24::AID-ARCH3>3.0.CO;2-H/abstract. |
| [24] | YAN J, CHENG Q, NARASHIMHAN S, et al. Cloning and functional expression of a fat body-specific chitinase cDNA from the tsetse fly, Glossina morsitans morsitans [J/OL]. Insect Biochem Molec Biol, 2002, 32(9):979-989[2015-08-09].http://www.sciencedirect.com/science/article/pii/S0965174802000346. doi:10.1016/S0965-1748(02)00034-6. |
| [25] | SHINODA T, KOBAYASHI J, MATSUI M, et al. Cloning and functional expression of a chitinase cDNA from the common cutworm, Spodoptera litura, using a recombinant baculovirus lacking the virus-encoded chitinase gene[J/OL]. Insect Biochem Molec, 2001, 31:521-532[2015-08-09].http://www.sciencedirect.com/science/article/pii/S0965174800001338. doi:10.1016/S0965-1748(00)00133-8. |
| [26] | YANG W J, XU K K, ZHANG R Y, et al. Transcriptional regulation of a chitinase gene by 20-hydroxyecdysone and starvation in the oriental fruit fly, Bactrocera dorsalis [J/OL]. Int J Mol Sci, 2013, 14(10):20048-20063[2015-08-10].http://www.mdpi.com/1422-0067/14/10/20048/htm. doi:10.3390/ijms141020048. |
| [27] | ZHU Q S, ARAKANE Y, BEEMAN R W, et al. Functional specialization among insect chitinase family genes revealed by RNA interference[J/OL]. P Nati Acad Sci USA, 2008, 105(18):6650-6655[2015-08-10]. http://www.pnas.org/content/105/18/6650.short. doi:10.1073/pnas.0800739105. |
| [28] | ZHANG J Z, ZHANG X, ARAKANE Y, et al. Comparative genomic analysis of chitinase and chitinase-like genes in the African malaria mosquito(Anopheles gambiae)[J/OL]. PLoS One, 2011, 6(5):e19899[2015-08-10].http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0019899. |
| [29] | MERZENDORFER H. Insect-derived chitinases[J/OL]. Adv Biochem Eng Biotechnol, 2013, 136:19-50[2015-08-10]. http://link.springer.com/chapter/10.1007/10_2013_207. doi:10.1007/978-3-642-39902-2. |
| [30] | 丁伟.螨类控制剂[M]. 北京:化学工业出版社, 2011:184-185.DING W. Mite control agent[M]. Beijing:Chemical Industry Press, 2011:184-185. |
| [31] | 师光禄, 王有年, 王鸿雷, 等. 万寿菊根提取物对山楂叶螨谷胱甘肽S-转移酶和蛋白酶及蛋白质含量的影响[J]. 应用生态学报, 2007, 18(2):400-404.SHI G L, WANG Y N, WANG H L, et al. Effects of Tagetes erecta extracts on glutathione S-transferase and protease activities and protein content in Tetranychus viennensis [J]. Chin J Appl Ecol, 2007, 18(2):400-404. |
 2016, Vol. 18
2016, Vol. 18


