褐飞虱Nilaparvata lugens Stl是我国和许多亚洲国家水稻生产上的首要害虫,具有远距离迁飞习性,其内禀增长率高,环境适应能力强,外界条件适宜时极易暴发成灾[1, 2]。近年来,随着耕作技术的改变和高产杂交稻的大面积推广,褐飞虱的危害日益加重。在我国褐飞虱每年为害的水稻种植面积超过4×106 hm2,仅2005和2008年直接危害造成的稻谷损失就达2.7×109 kg[3]。目前,化学杀虫剂是控制褐飞虱的主要途径,但大量使用不仅增加生产成本,而且杀伤天敌、增加褐飞虱抗药性,引起褐飞虱的再猖獗[4]。因此,对褐飞虱的防治宜采用以生物防治为主的综合治理措施[5]。
昆虫的病原微生物是害虫自然控制和生物防治的重要组成部分,我国已报道的有关防治水稻害虫的病原微生物有64种,其中真菌36种,细菌5种,病毒6种,微孢子虫2种,线虫15种[6]。通过采集自然感病褐飞虱,已分离获得其生防真菌黄绿绿僵菌和桔形被毛孢[5, 7, 8]。王艳秋等[9]从罹病二化螟中分离到对褐飞虱具有致病力的曲霉和白僵菌。但关于病原细菌防治褐飞虱的研究鲜有报道[6]。本研究从感病死亡的蔷薇蚜虫体内分离筛选鉴定出一株对褐飞虱具有较高致病力的细菌菌株,并对其细菌发酵液和细胞破碎液进行了杀虫活性测定,以期为应用杀虫细菌防治褐飞虱提供理论依据。
1 材料与方法 1.1 病虫来源2014年从江苏省农业科学院内蔷薇上采集感病死亡的蔷薇蚜虫,置于4 ℃冰箱保存,备用。
1.2 供试昆虫试验用褐飞虱种群采自江苏省浦口区稻田,在温室(26 ℃±1 ℃,光照L∶D=12∶12) 内用稻苗(武运粳3号)连续饲养2年以上。
1.3 病原菌的分离、纯化和回复感染 1.3.1 病原菌的分离及纯化 1.3.2 病原菌的初筛和回复感染将分离、纯化出的菌株接种于液体发酵培养基中[12],经48 h摇瓶培养后喷洒到水稻苗上,供褐飞虱3龄若虫取食感染,收集具有典型病症的虫体;将病虫体置于70%乙醇中浸泡5 s后,用无菌水清洗3~5次,移入无菌水试管中,用无菌玻璃棒轻压碎虫体,使黑褐色体液流出配制成101、102和103 CFU/mL系列菌悬液;在无菌条件下取0.1 mL菌悬液涂布于LB固体平板培养基上,于30 ℃下培养48 h 后,挑取不同单孢菌落到新的LB平板上培养。
将分离纯化的菌株再次接种于液体发酵培养基上,重复上述步骤,确定菌株对褐飞虱的致病性,收集具有典型病症的虫体。再次分离、纯化菌株后,转到LB培养基上,置于4 ℃冰箱保存,备用。
1.4 致病菌株的鉴定将筛选出的致病菌株接种于LB平板上活化,培养36 h左右进行革兰氏染色;参照Sun等的方法[13]进行生长温度和耐盐度的测定,同时挑取单个菌落接种于微量生化反应管中,于35 ℃下培养48 h,用于进行酶活性检测、吲哚和 V-P 试验以及糖醇发酵试验,观察结果,对菌株进行初步鉴定。
菌株的分子生物学鉴定参考Mostakim 等的方法进行[14]。利用细菌16S rDNA的通用引物27F和1492R进行菌落PCR 扩增,其中正向引物27F的序列为5′-AGAGTTTGATCATGGCTCAG-3′,反向引物1492R的序列为5′-TACGGYTACCTTGTTACGACTT-3′。PCR 反应体系:30 μL的反应体系中含模板DNA 2 μL、dNTP 1 μL、正反向引物各1 μL、10 PCR Buffer 3 μL、Taq 酶(2 U/ μL)1 μL,用ddH2O补足到30 μL。循环条件:95 ℃预变性5 min后进入循环;95 ℃变性30 s、57 ℃退火30 s、72 ℃延伸1.5 min,30个循环后,72 ℃延伸3 min。PCR产物的扩增纯化和测序均由南京金斯瑞有限公司完成。将获得的菌株16S rDNA 基因序列提交到 NCBI基因库(GenBank)进行BLAST比对。用 MEGA5.0 软件以邻接法(Neighbor-Joining 法)构建系统发育树。发育树的构型和稳定性用MEGA5.0软件取样分析1 000次,进行Bootstrap值分析和评价。
1.5 盆栽试验挑取一环高致病力菌株的单菌落,置于5 mL LB液体培养基中,于30 ℃、250 r/min下培养12 h后,取1 mL转接入100 mL LB液体培养基,同样条件培养48 h,得到菌液。采用平板计数法确定菌液的浓度。然后用无菌水配制成106和108 CFU/mL的菌悬液(含0.03%吐温80),喷雾到培养50~60 d的水稻苗上(5株/盆),每盆喷雾量20 mL,然后接种50头褐飞虱3龄若虫。以含0.03%吐温80的无菌水作对照。每处理重复4次。分别于处理后24、48和72 h,观察记录死亡虫数。运用 SPSS 18.0 软件,采用单因素方差分析和 Tukey 多重比较检验各处理间的差异显著性。
1.6 粗提物的杀虫活性测定 1.6.1 细菌发酵液和细胞破碎液的制备参照杨琼等的方法[15]。取1.5节得到的108 CFU/mL菌悬液,于10 000 r/min下离心10 min。取上清液用0.22 μm无菌滤膜过滤,获得细菌发酵液;离心管管底的菌体沉淀重悬于PBS缓冲液,超声波破碎,用0.22 μm无菌滤膜过滤,获得细胞破碎液。
1.6.2 粗提物的杀虫活性测定采用稻苗浸渍法[16]测定细菌发酵液和细胞破碎液对褐飞虱3龄若虫的作用活性。取室内培养的长约10 cm的水稻苗,分别置于细菌发酵液和细胞破碎液中浸泡30 s,取出晾干,放入塑料杯(直径8 cm,高12 cm)中各接种20头大小一致的褐飞虱3龄若虫。分别以无菌水和PBS 缓冲液作为空白对照。处理组和对照组均含0.03%吐温80,每处理重复4次。分别于处理后24、48和72 h,观察记录死亡虫数。
2 结果与分析 2.1 菌株的分离及致病性测定将从感病蚜虫体中分离获得的菌株标记为JSA1,该菌株在LB液体培养基中,菌液呈暗绿色。稀释喷雾到水稻苗,然后接种健康褐飞虱若虫取食,感染该菌后虫体呈黑褐色(图1)。对感病的若虫再次分离培养发现,分离前后菌株的形态特征、培养性状等均一致,表明菌株JSA1为褐飞虱的致病菌。
 | 图 1 感染菌株JSA1的褐飞虱若虫症状 Fig. 1 Infection symptoms of strain JSA1 in N.lugens nymph |
由表1可知:菌株JSA1革兰氏染色呈阴性,在4 ℃时不生长,而在42 ℃下可生长。耐盐范围为3%~8%,能够产生绿脓菌素,甲基红、吲哚反应和 V-P 反应均呈阴性,山梨醇呈阳性,侧金盏花醇和甘露醇呈阴性;能水解赖氨酸、鸟氨酸和枸橼氨酸,不能将苯丙氨酸氧化脱氨,不能将葡萄糖、蔗糖等发酵产酸,含有脲酶,不产生硫化氢。
|
|
表 1 菌株JSA1的主要生理生化特征 Table 1 Physiological and biochemical properties of strain JSA1 |
以菌株JSA1的基因组DNA为模板,PCR扩增产物的序列长为1 381 bp(图2)。将扩增序列在GenBank中进行BLAST相似性比对,结果表明菌株JSA1与假单胞菌属的铜绿假单胞菌同源性最高。利用 MEGA5.0软件,将菌株JSA1的16S rDNA序列分别与GenBank中已登录的11种假单胞菌属的16S rDNA 序列构建系统发育树。由图3可以看出:菌株JSA1与Pseudomonas aeruginosa (GenBank登录号为HQ268732)聚成一群,相似性≥99%,结合菌株的形态特征以及生理生化特性,初步确定该菌株为铜绿假单胞菌P. aeruginosa。
 | M:DL3000 marker;泳道1:PCR产物。 M: DL3000 marker; Lane 1: PCR product. 图 2 菌株JSA1 16S rDNA的PCR扩增结果 Fig. 2 PCR amplification product of strain JSA1 16S rDNA |
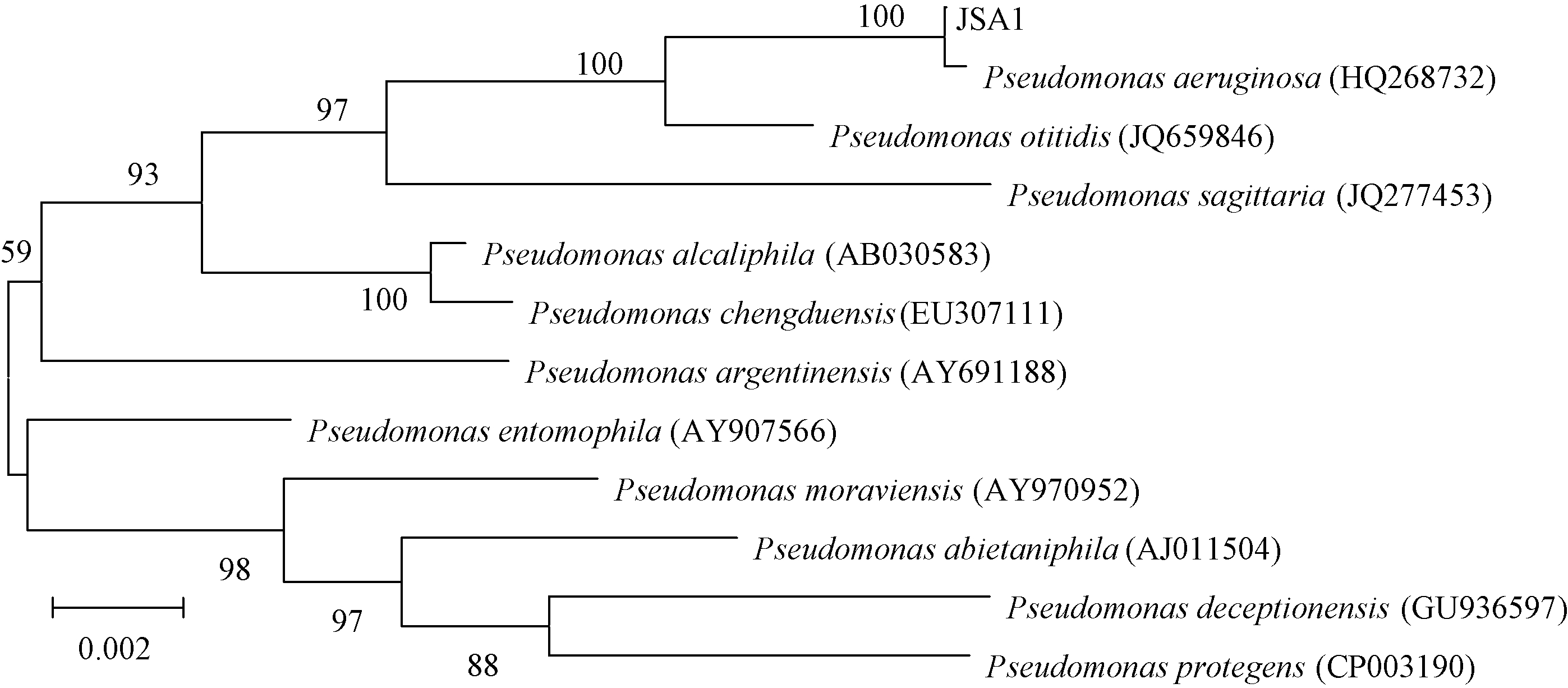 | 图 3 菌株JSA1 16S rDNA 序列系统发育树 Fig. 3 Molecular phylogenetic tree of strain JSA1 based on 16S rDNA sequences |
图4结果显示:不同浓度菌悬液对褐飞虱3龄若虫均有杀虫作用,且致死率随菌液浓度的升高和作用时间的延长而提高,但杀虫效果低于40%。48和72 h的处理组与对照间均存在显著差异。
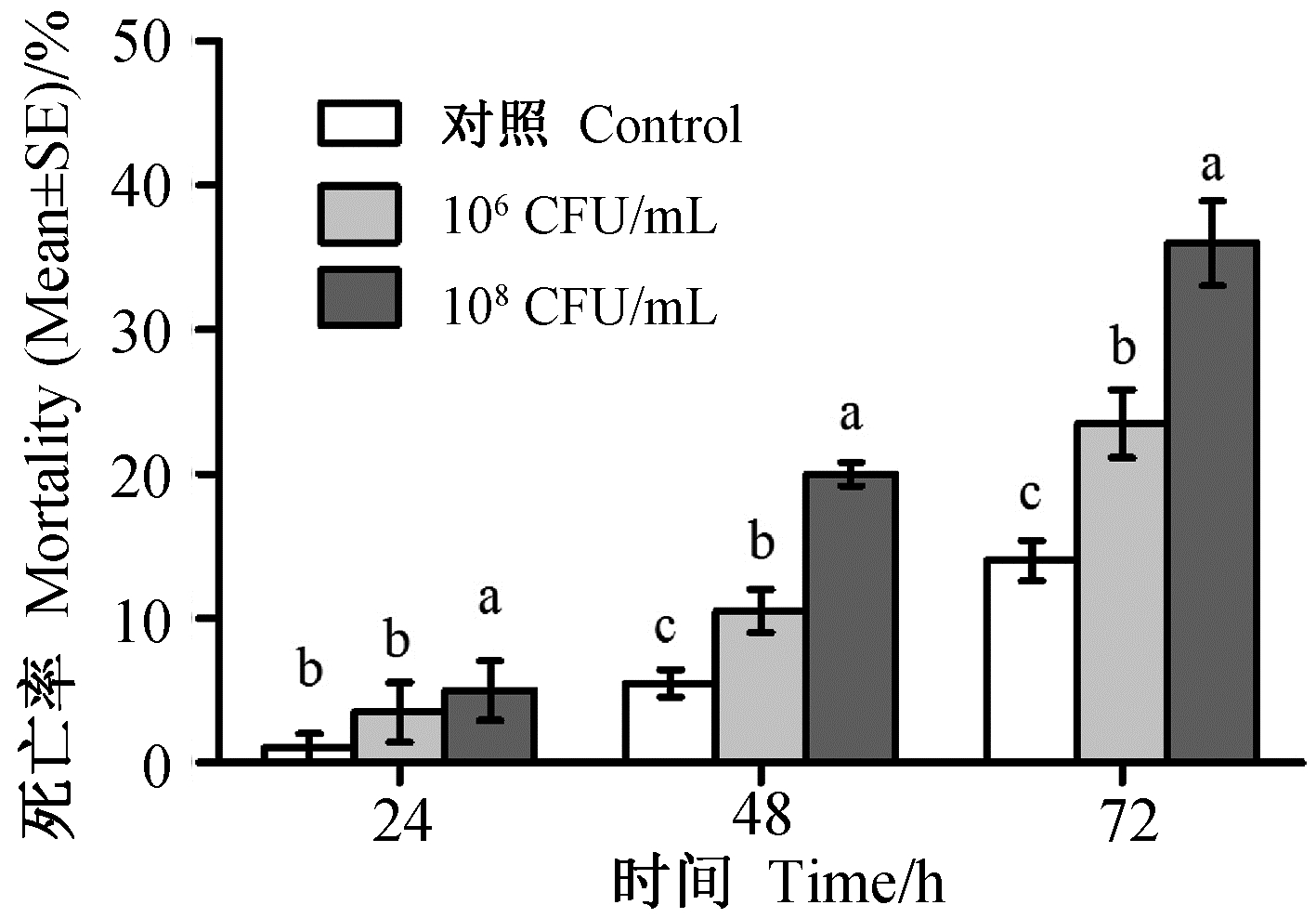 | 图 4 JSA1菌悬液处理褐飞虱3龄若虫死亡率 Fig. 4 Mean mortality of the 3rd instar nymphs of N.lugens after treatment with strain JSA1 |
菌株JSA1的发酵液和细胞破碎液对褐飞虱3龄若虫均具有杀虫活性,但细胞破碎液的杀虫效果更好。在处理后24、48和72 h,发酵液对褐飞虱3若虫的校正死亡率分别为7.63%、20.39%和30.72%;细胞破碎液对褐飞虱的校正死亡率分别为66.64%、70.69%和82.53%。这说明该菌胞外和胞内均含有杀虫活性物质,而且主要存在于胞内(图5)。
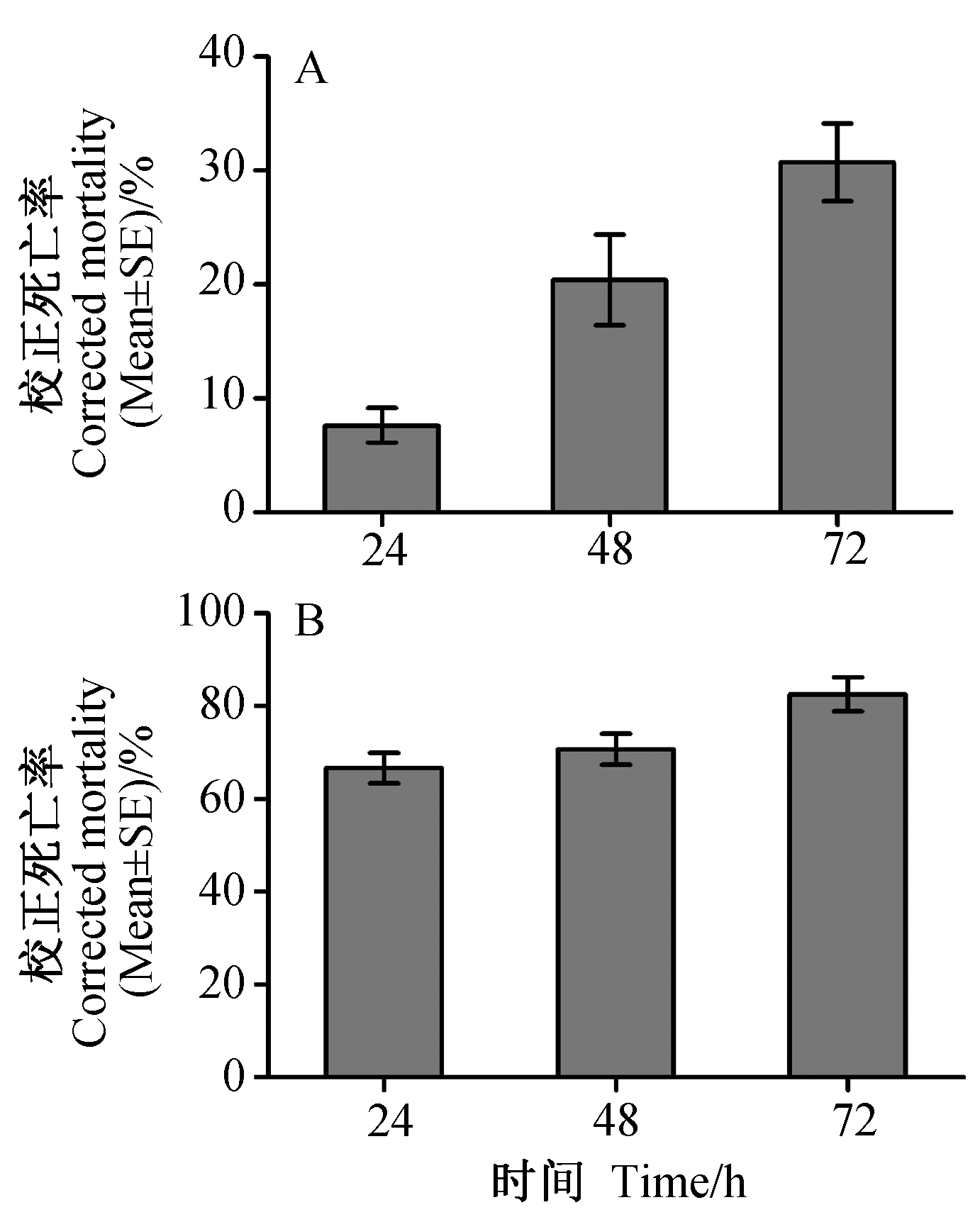 | 图 5 菌株JSA1的发酵上清液(A)和细胞破碎液(B)对褐飞虱3龄若虫的杀虫活性 Fig. 5 Insecticidal activities of supernatant of fermentation broth (A) and suspension of sonicated cell pellet (B) of strain JSA1 against the 3rd instar nymphs of N. lugens |
近年来褐飞虱曾多次暴发成灾,给水稻生产造成严重损失[1, 2, 3, 17]。在江苏省沿江、苏南、丘陵及淮北局部地区的田间调查结果显示,田间褐飞虱虫卵量呈明显上升趋势[18]。 长期以来,化学防治一直是控制褐飞虱的重要途径,导致褐飞虱对氟虫腈、吡虫啉和噻嗪酮等产生了较高的抗性[19]。因此,利用感病昆虫分离和鉴定褐飞虱致病菌株,对于解决褐飞虱的防治问题具有重要意义。Lau等[20]报道了铜绿假单胞菌对果蝇的杀虫活性;Mostakim等[14]发现铜绿假单胞菌IL5对橄榄果蝇具有较高的生防潜力,其代谢产物对3龄幼虫的致死率达100%。同类菌株荧光假单胞菌(P.florescens)、嗜虫假单胞菌(P.entomophila)、绿针假单胞菌(P.chlororaphis)等对农业害虫也具有较好的杀虫活性,而且基于假单胞菌属菌株已开发出杀菌剂Cedomon和Cerall[21]。本研究也发现菌株JSA1的胞内和胞外均存在杀虫活性物质,尤其是胞内物质活性更高,因此,对褐飞虱具有较好生防潜力的菌株JSA1,可望下一步利用其防治水稻害虫。
利用昆虫致病微生物的代谢产物防治植物病虫害的研究已受到广泛关注[20, 21, 22, 23]。苏云金芽孢杆菌的Cry和Cyt毒素[22],初生型发光杆菌属及嗜线虫致病杆菌属的Tc毒素[23],白僵菌和绿僵菌的胞外杀虫蛋白均表现出较好的杀虫活性[24, 25]。从假单胞菌中已分离到的具有杀虫活性的次级代谢产物有oxazoles和鼠李糖脂等[26, 27];从铜绿假单胞菌PA14中分离到吩嗪对秀丽杆线虫具有杀虫活性[28]。但微生物的代谢产物受多种因素影响,不同种或同种不同菌株产生的胞内或胞外物质的杀虫活性不同,同一菌株也会因培养条件的不同而影响杀虫活性物质的产生或产量。本研究中,菌株JSA1的杀虫活性究竟是何种物质成分在起作用尚需进一步研究。
从环境中分离昆虫致病性微生物是目前害虫生物防治的重要途径,也是微生物农药开发利用的基础[29],但其生物安全性问题不容忽视。对于从自然界分离获得的杀虫菌株,在生产应用前,即使菌株具有较强的杀虫活性,也要充分评估其对人类和环境的影响[30]。目前,美国、加拿大、荷兰、日本、韩国和南非等国家都构建了微生物农药安全性评价技术体系[31];我国虽未建立专门的微生物农药安全性评价技术与相关试验导则,但要申请登记微生物农药也需提供急性毒性试验、药效试验、产品化学、生物学特性、毒理学、环境影响以及境外登记情况等资料[31]。因此,虽然铜绿假单胞菌对褐飞虱具有较高的生防潜力,尤其是细胞破碎液,但其安全性同样需要充分评估,以便合理的推广应用。
| [1] | Gurr G M, Liu J, Read D M Y, et al. Parasitoids of Asian rice planthopper (Hemiptera: Delphacidae) pests and prospects for enhancing biological control by ecological engineering [J]. Ann Appl Biol, 2011, 158(2): 149-176. |
| [2] | Bottrell D G, Schoenly K G. Resurrecting the ghost of green revolutions past: the brown planthopper as a recurring threat to high-yielding rice production in tropical Asia [J]. J Asia Pac Entomol, 2012, 15(1): 122-140. |
| [3] | Brar D S, Virk P S, Jena K K, et al. Breeding for resistance to planthoppers in rice [M]//Heong K L, Hardy B. Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia. Los Baåos, Philippines: International Rice Research Institute, 2009: 401-428. |
| [4] | Zhang Xiaolei, Liu Xiangyang, Zhu Fuxing, et al. Field evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) in China [J]. Crop Prot, 2014, 58: 61-66. |
| [5] | 李茂业,林华峰,李世广,等.褐飞虱高毒力绿僵菌菌株的筛选 [J].菌物学报, 2012, 31(3): 331-340. Li Maoye, Lin Huafeng, Li Shiguang, et al. Screening of high virulent strain of Metarhizium spp. against the brown planthopper, Nilaparvata lugens [J]. Mycosystema, 2012, 31(3): 331-340. (in Chinese) |
| [6] | Lou Yonggen, Zhang Guren, Zhang Wenqing, et al. Biological control of rice insect pests in China [J]. Biol Control, 2013, 67(1): 8-20. |
| [7] | Li Maoye, Li Shiguang, Xu Amei, et al. Selection of Beauveria isolates pathogenic to adults of Nilaparvata lugens [J]. J Insect Sci, 2014, 14(1): 32. |
| [8] | 贾春生,洪波.一种潜在的褐飞虱生防真菌:桔形被毛孢的分离、鉴定与培养 [J].东北农业大学学报, 2010, 41(7): 27-31. Jia Chunsheng, Hong Bo. A potential fungus for Nilaparvata lugens biocontrol: isolation, identification and culture of Hirsutella citriformis [J]. J Northeast Agric Univ, 2010, 41(7): 27-31. (in Chinese) |
| [9] | 王艳秋,洪勇,岳霄霄,等.二化螟幼虫病原真菌的分离纯化及其对烟粉虱和褐飞虱的毒力研究 [J].环境昆虫学报, 2014, 36(6): 943-950. Wang Yanqiu, Hong Yong, Yue Xiaoxiao, et al. Study on the purification of pathogenic fungi from the rice stem borer larvae and the control of Bemisia tabaci and Nilaparvata lugens [J]. J Environ Entomol, 2014, 36(6): 943-950. (in Chinese) |
| [10] | 沈平,范秀容,李广武.微生物学实验[M]. 3版.北京:高等教育出版社, 1999. Shen Ping, Fan Xiurong, Li Guangwu. Experiments of microbiology[M]. 3rd ed. Beijing: Higher Education Press, 1999. (in Chinese) |
| [11] | 王磊,张灼,欧晓昆,等.细菌在蚜虫生物防治中的研究初报 [J].广西农业科学, 2010, 41(3): 226-230. Wang Lei, Zhang Zhuo, Ou Xiaokun, et al. Preliminary researches on the aphides bio-control with bacteria [J]. Guangxi Agric Sci, 2010, 41(3): 226-230. (in Chinese) |
| [12] | Heo Y J, Lee Y R, Jung H H, et al. Antibacterial efficacy of phages against Pseudomonas aeruginosa infections in mice and Drosophila melanogaster.Antimicrob Agents Chemothe, 2009,53(6):2469-2474. |
| [13] | Sun Yamin, Wang Min, Liu Hongbo, et al. Development of an O-antigen serotyping scheme for Cronobacter sakazakii [J]. Appl Environ Microbiol, 2011, 77(7): 2209-2214. |
| [14] | Mostakim M, Soumya E, Mohammed I H, et al. Biocontrol potential of a Pseudomonas aeruginosa strain against Bactrocera oleae [J]. Afr J Microbiol Res, 2012, 6(26): 5472-5478. |
| [15] | 杨琼,周宇,韩光杰,等.两种甜菜夜蛾致病菌的分离、鉴定及杀虫成分初步分析[J].江苏农业学报, 2013, 28(6): 1267-1271. Yang Qiong, Zhou Yu, Han Guangjie, et al. Isolation, identification and active ingredient analysis of two pathogenic bacterial strains isolated from Spodoptera exigua [J]. Jiangsu J Agric Sci, 2013, 28(6): 1267-1271. (in Chinese) |
| [16] | 谢化鹏,宋宝安,金林红,等.噻嗪酮、烯啶虫胺及其复配制剂对褐飞虱3龄若虫的毒力测定 [J].农药, 2010, 49(1): 74-77. Xie Huapeng, Song Baoan, Jin Linhong, et al. Toxicity measure of buprofezin, nitenpyram and their mixtures to the third instar nymphs of brown planthopper [J]. Agrochemicals, 2010, 49(1): 74-77. (in Chinese) |
| [17] | Hu Gao, Lu Fang, Zhai Baoping, et al. Outbreaks of the brown planthopper Nilaparvata lugens (Stl) in the Yangtze River Delta: immigration or local reproduction? [J]. PLoS One, 2014, 9(2): e88973. |
| [18] | 江苏省植保站.褐飞虱虫卵量上升防治不容忽视 [N].江苏农业科技报, 2012-09-15. Jiangsu Plant Protection Station. The increased number of Nilaparvata lugens eggs, prevention and control cannot be ignored [N]. Jiangsu Agric Sci Technol Paper, 2012-09-15. (in Chinese) |
| [19] | 王鹏,甯佐苹,张帅,等.我国主要稻区褐飞虱对常用杀虫剂的抗性监测 [J].中国水稻科学, 2013, 27(2): 191-197. Wang Peng, Ning Zuoping, Zhang Shuai, et al. Resistance monitoring to conventional insecticides in brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) in main rice growing regions in China [J]. Chin J Rice Sci, 2013, 27(2): 191-197. (in Chinese) |
| [20] | Lau G W, Goumnerov B C, Walendziewicz C L, et al. The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa [J]. Infect Immun, 2003, 71(7): 4059-4066. |
| [21] | Kupferschmied P, Maurhofer M, Keel C. Promise for plant pest control: root-associated pseudomonads with insecticidal activities [J]. Front Plant Sci, 2013, 4: 287. |
| [22] | Bravo A, Likitvivatanavong S, Gill S S, et al. Bacillus thuringiensis: a story of a successful bioinsecticide [J]. Insect Biochem Molec Biol, 2011, 41(7): 423-431. |
| [23] | ffrench-Constant R H, Dowling A, Waterfield N R. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture [J]. Toxicon, 2007, 49(4): 436-451. |
| [24] | 臧欢,刘廷辉,李瑞军,等.高毒力金龟子绿僵菌的筛选及其胞外蛋白酶产量测定[J].农药学学报, 2013, 15(2): 183-187. Zang Huan, Liu Tinghui, Li Ruijun, et al. Screening for high virulence strains of Metarhizium anisopliae and yield determination of its extracellular protease [J]. Chin J Pestic Sci, 2013, 15(2): 183-187. (in Chinese) |
| [25] | 刘智辉,陈守文,郭志红,等.球孢白僵菌胞外蛋白酶和几丁质酶活性与对亚洲玉米螟毒力的相关性分析 [J].华中农业大学学报, 2005, 24(4): 364-368. Liu Zhihui, Chen Shouwen, Guo Zhihong, et al. Correlation of extracellaluar protease, chitinase activities and virulence to Ostrinia furnacali [J]. J Huazhong Agric Univ, 2005, 24(4): 364-368. (in Chinese) |
| [26] | Grundmann F, Dill V, Dowling A, et al. Identification and isolation of insecticidal oxazoles from Pseudomonas spp. [J]. Beilstein J Org Chem, 2012, 8(1): 749-752. |
| [27] | Kim S K, Kim Y C, Lee S, et al. Insecticidal activity of rhamnolipid isolated from Pseudomonas sp. EP-3 against green peach aphid (Myzus persicae) [J]. J Agric Food Chem, 2010, 59(3): 934-938. |
| [28] | Cezairliyan B, Vinayavekhin N, Grenfell-Lee D, et al. Identification of Pseudomonas aeruginosa phenazines that kill Caenorhabditis elegans [J]. PLoS Pathog, 2013, 9(1): e1003101. |
| [29] | Ruiu L, Satta A, Floris I. Emerging entomopathogenic bacteria for insect pest management [J]. Bull Insectol, 2013, 66(2): 181-186. |
| [30] | 李荣森.我国微生物防治研究与微生物农药产业化的进展(1980—1999) [J].中国病毒学, 2000, 15(增刊): 1-15. Li Rongsen. Microbial control of pests and industrialization of microbial pesticides in China (1980-1999)[J]. Virol Sinica, 2000, 15(Suppl.1): 1-15. (in Chinese) |
| [31] | 陈源,卜元卿,单正军.微生物农药研发进展及各国管理现状[J].农药, 2012, 51(2): 83-89. Chen Yuan, Bu Yuanqing, Shan Zhengjun. Reviews on development and management of microbial pesticide in different countries [J]. Agrochemicals, 2012, 51(2): 83-89. (in Chinese) |
 2015, Vol. 17
2015, Vol. 17


 , 郭慧芳
, 郭慧芳