2. 植物病虫害生物学国家重点实验室, 中国农业科学院植物保护研究所, 北京 100193
2. State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, China
Esters are ubiquitous and common bioactive. For example,phosphoesters form the backbone of DNA molecules. Esters with low molecular weight are usually found in essential oils and pheromones. Many esters have distinctive odors,and many of them occur naturally in the essential oils of plants. This has also led to their safe use in artificial flavorings and fragrances when those odors aim to be mimicked.
Pyrethroids[1] are one of the most famous agrochemicals included in over 3 500 registered products,many of which are widely used in and around households,including on pets,in mosquito control,and in agriculture. Carbamates[2,3] are another world wide used pesticide. Both of them contained and connected with the ester moieties (Fig. 1A,1B and 1C). In our previous study,some thymol and carvacrol derivatives containing 5-phenyl-2-furan were designed and synthesized,which was linked by ester moieties (Fig. 1D and 1E). These compounds possessed good antitumor and fungicidal activity[4].
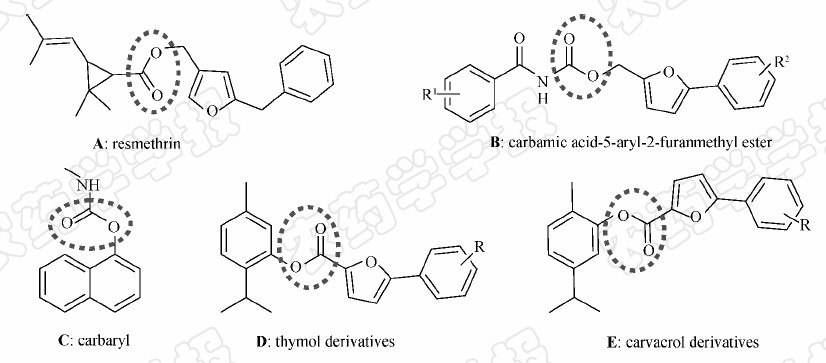 | Fig. 1 Bioactive compounds with ester moieties |
Pyridazin-3(2H)-one derivative is well known as the important scaffolds in drugs and agrochemicals[5,6,7,8,9,10],which is a good synthetic building block for constructing new bioactive compounds due to their easy-functionalization at various ring positions[11,12,13]. So the phenyl ring in our former compounds was replaced with the pyridazin-3(2H)-one (Fig. 2) and a series of novel 5-ester-pyridazin-3(2H)-one derivative with 5-phenyl-2-furan moiety were designed and synthesized (Scheme 1). Their fungicidal activity was evaluated.
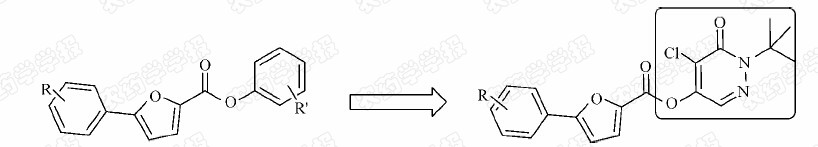 | Fig. 2 The designed strategy for the title compounds |
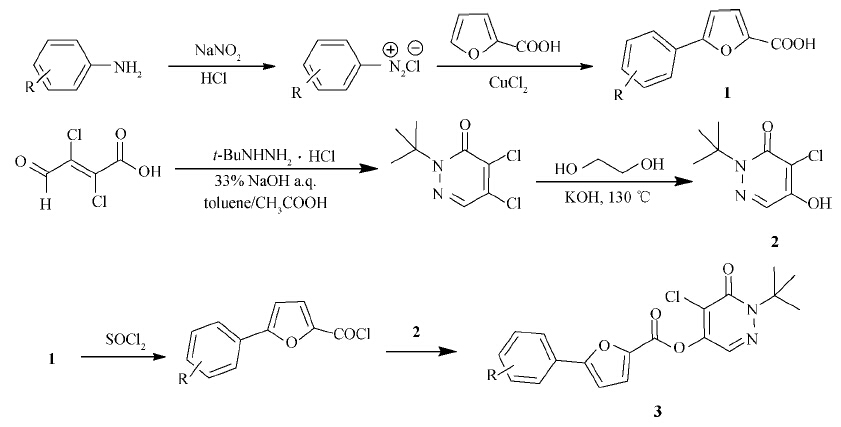 | Scheme 1General synthetic procedure for title compounds 3a-3o |
All the melting points were determined with a Cole-Parmer melting point apparatus while the thermometer was uncorrected. IR spectra were recorded on a NEXUS-470 FTIR (Nicolet) spectrometer with KBr pellets.1H NMR spectra were recorded with Bruker AVANCE III 400 instrument,while tetramethylsilane was used as an internal standard. Analytical thin-layer chromatography (TLC) was carried out on precoated plates (silica gel 60 F254),and spots were visualized with ultraviolet (UV) light. Elemental analysis was carried out with a Flash EA 1112 elemental analyzer.
The key intermediate 5-substituted phenyl-2-furancarboxylic acid 1 was synthesized from substituted aniline by Meerwein arylation reaction according to the reported procedure[14,15,16,17,18].
t-Butyl hydra-zine hydrochloride (0.1 mol) was mixed with 33% sodium hydroxide (0.1 mol) solution and 100 mL toluene. Then mucochloric acid (0.11 mol) and acetic acid (0.1 mol) were added into the solution. The mixture was stirred at 40 ℃ for 4 h. After that,the organic layer was washed with 33% sodium hydroxide solution,35% hydrochloric acid and brine,dried over MgSO4 and concentrated in vacuo to afford light yellow crystal,yield 80%,m.p. 63-64 ℃.1H NMR (300 MHz,CDCl3),δ: 1.65 (s,9H,t-C4H9),7.73 (s,1H,CH=N).
(2H)-one (2) 2-tert-Butyl-4,5-dichloropyridazin-3(2H)-one (0.1 mol) and KOH (0.5 mol) were dissolved in 300 mL glycol,and stirred at 130 ℃ for 5 h. The reaction was quenched by addition of ice water and the solution was acidified to pH 1.0 by hydrochloric acid. The solid was washed with water and dried to afford compound 2. Yield 96%,m.p. 219-220 ℃.1H NMR (400 MHz,DMSO-d6),δ: 1.65 (s,9H,t-C4H9),7.80 (s,1H,N=CH),8.97 (s,1H,OH). Anal. Calcd. (%) for C8H11ClN2O2: C,47.42; H,5.47; N,13.82. Found: C,47.59; H,5.31; N,14.01.
A mixture of 5-substituted phenyl-2-furancarboxylic acid (0.05 mol) and thionyl chloride (0.15 mol) was refluxed in anhydrous benzene for 3 h. The excess of thionyl chloride and the solvent were distilled off,and the residue was dissolved in anhydrous benzene. The solution of 5-substituted phenyl-2-furancarbonyl chloride in anhydrous benzene was added into 5-hydroxy-pyridazin-3(2H)-one. The mixture was stirred and refluxed for 5 h. After cooling,the solvent was evaporated off under reduced pressure,and the solid was recrystallized from ethyl acetate to obtain the title compounds 3a-3o.
Fungicidal activity of the title compounds against Botrytis cinerea,Corynespora cassiicola,Fusarium oxysporum and Rhizoctonia solanii were evaluated using mycelium growth rate test[19,20,21] at 50 mg/L. The fungi were provided by Institute of Plant Protection,CAAS,Beijing,China. All the strains were maintained on potato dextrose agar (PDA) medium at 4 ℃. Commercial fungicide procymidone was as control against the above mentioned fungi under the same condition. Three replicates were performed. The relative inhibition rate of the circle mycelium compared to blank assay was calculated via the following equation:
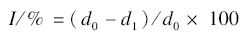
In which,I stands for the inhibition (%), d0 is the diameter of mycelia in the blank control test (in mm),and d1 is the diameter of mycelia in the presence of tested compounds (in mm).
Physical and chemical data of the title compounds 3a-3o were listed in Table 1.1H NMR and IR spectra were listed in Table 2.
| Table 1 Physical and chemical data of title compounds 3a-3o |
| Table 2 1H NMR and IR spectra of title compounds 3a-3o |
Four kinds of plant pathogens, B. cinerea,C. cassiicola,F. oxysporum,and R. solani,were chosen for bioassay. As shown in Table 3,most of the compounds exhibited considerable fungicidal activity in vitro against the above four strains at 50 mg/L.
| Table 3 Fungicidal activity of title compounds 3a-3o at 50 mg/L |
The fungicidal activity of title compounds against four test fungi could draw some conclusions as follows: (a) Compounds 3i had a significant activity against B. cinerea,and its fungicidal activity of 89.16%±1.73% was equal to that of the fungicide procymidone (88.58%±1.64%). The fungicidal activity of compounds 3c,3g and 3k against B. cinerea was more than 60%. (b) For C. cassiicola and F. oxysporum,compound 3k showed the best control efficacy of 70.91%±1.26% and 77.28%±1.56% respectively,which was a little bit lower than the procymidone (79.06%±1.57% and 85.47%±1.04%). Compounds 3g and 3i also gave inhibitory rates of 67.29%±1.65% and 66.49%±1.18% to the C. cassiicola. The inhibitory rate of 3i to the F. oxysporum was 60.19%±1.26%. (c) Among all of the title compounds,compounds 3i and 3k displayed the best effects of 81.27%±1.38% and 79.38%±1.24% against R. solani,which was the same as the procymidone (79.62%±1.15%). The preliminary structure-activity relationship showed that the difference of R group affected the fungicidal activity. When R was NO2,they gave better activity than that of F,Cl,Br,CH3 and OCH3. When R was at the ortho or para position,the compounds displayed higher fungicidal activity than that of at the meta position. Compound 3i showed great promise as a lead compound for further antifungal discovery.
| [1] | LIU S Z, WANG M, CHEN F H. Research progress and development prospect of pyrethroid pesticide[J]. Chin J Pesitic, 2004, 43(7): 289-293 (in Chinese) |
| [2] | ADAMS P, BARON F A. Esters of carbamic acid[J]. Chem Rev, 1965, 65(5): 567-602. |
| [3] | LI Y, LI B J, LING Y, et al. Synthesis and fungicidal activity of aryl carbamic acid-5-aryl-2-furanmethyl ester[J]. J Agric Food Chem, 2010, 58(5): 3037-3042. |
| [4] | CUI Z N, LI X H, NISHIDA Y. Synthesis and bioactivity of novel carvacrol and thymol derivatives containing 5-phenyl-2-furan[J]. Lett Drug Des Discov, 2014: (DOI: 10.2174/1570180811666140220005252). |
| [5] | LING Y, CHEN H F, LIU J, et al. Synthesis and biological activity of 5-(acyl)hydrazine-3(2H)-pyridazinone[J]. Chin J Pestic Sci, 2009, 11(1): 25-30. (in Chinese) |
| [6] | LING Y, ZHU W W, SHI W P, et al. Synthesis and insect growth regulating activity of 5-benzoylphenyl urea 3(2H)-pyridazinone[J]. Chin J Pestic Sci, 2008, 10(2): 141-146. (in Chinese) |
| [7] | LING Y, LI H S, YANG X L, et al. Design, synthesis and biological activity of 5-(alkoxy)-3(2H)-pyridazinone derivatives[J]. Chin J Org Chem, 2007, 27(6): 763-767. (in Chinese) |
| [8] | LI H S, LING Y, GUO Y L, et al. Study on the synthesis and bioactivity of pyridazinone derivatives[J]. Chin J Org Chem, 2005, 25(2): 204-207. (in Chinese) |
| [9] | CAO S, WEI N, ZHAO C M, et al. Syntheses, antifeedant activity, and QSAR analysis of new oxa(thia)diazolyl 3(2H)-pyridazinones[J]. J Agric Food Chem, 2005, 53(8): 3120-3125. |
| [10] | CAO S, QIAN X H, SONG G H, et al. Synthesis and antifeedant activity of new oxadiazolyl 3(2H)-pyridazinones[J]. J Agric Food Chem, 2003, 51(1): 152-155. |
| [11] | BANSAL R, THOTA S. Pyridazin-3(2H)-ones: the versatile pharmacophore of medicinal significance[J]. Med Chem Res, 2013, 22(6): 2539-2552. |
| [12] | SUNG G H, KIM B R, LEE S G, et al. 2-Substituted-pyridazin-3(2H)-ones as green electrophilic agents in synthesis[J]. Curr Org Chem, 2012, 16(7): 852-858. |
| [13] | WU J, SONG B A, HU D Y, et al. Research advance in insecticidal and acaricidal activities of pyridazine derivatives[J]. Agrochemicals, 2011, 50(9): 630-634. (in Chinese) |
| [14] | CUI Z N, YANG X L, SHI Y X, et al. Molecular design, synthesis and bioactivity of glycosyl hydrazine and hydrazone derivatives: Notable effects of the sugar moiety[J]. Bioorg Med Chem Lett, 2011, 21(23): 7193-7196. |
| [15] | CUI Z N, ZHANG L, HUANG J, et al. Synthesis and bioactivity of novel N,N'-diacylhydrazine derivatives containing furan(III)[J]. Chin J Chem, 2010, 28(7): 1257-1266. |
| [16] | CUI Z N, ZHANG L, HUANG J, et al. Synthesis, insecticidal activity and 3D-QSAR studies on diacylhydrazine derivatives containing furan[J]. Chin J Org Chem, 2010, 30(10): 1482-1491. (in Chinese) |
| [17] | CUI Z N, HUANG J, LI Y, et al. Synthesis and bioactivity of novel N,N'-diacylhydrazine derivatives containing furan(I)[J]. Chin J Chem, 2008, 26(5): 916-922. |
| [18] | CUI Z N, WANG Z, LI Y, et al. Synthesis of 5-(chlorophenyl)-2-furancarboxylic acid 2-(benzoyl)hydrazide derivatives and determination of their insecticidal activity[J]. Chin J Org Chem, 2007, 27(10): 1300-1304. (in Chinese) |
| [19] | LI X H, PAN Q, CUI Z N, et al. Synthesis and fungicidal activity of N-(2,4,5-trichlorophenyl)-2-oxo-and 2-hydroxycycloalkylsulfonamides[J]. Lett Drug Des Discov, 2013, 10(4): 353-359. |
| [20] | LI X H, CUI Z N, CHEN X Y, et al. Synthesis of 2-acyloxycyclohexyl-sulfonamides and evaluation on their fungicidal activity[J]. Int J Mol Sci, 2013, 14(11): 22544-22557. |
| [21] | CUI Z N, SHI Y X, ZHANG L, et al. Synthesis and fungicidal activity of novel 2,5-disubstituted-1,3,4-oxadiazole derivatives[J]. J Agric Food Chem, 2012, 60(47): 11649-11656. |
 2014, Vol.16
2014, Vol.16 


