文章信息
- 梅文晴, 冯宇妍, 姚志浩, 倪迎冬, 赵茹茜
- MEI Wenqing, FENG Yuyan, YAO Zhihao, NI Yingdong, ZHAO Ruqian
- 复合益生菌对肉仔鸡小肠形态和盲肠微生物菌群的影响
- Effects of compound probiotics supplementation on small intestinal morphology and cecal microflora in broiler chicks
- 南京农业大学学报, 2021, 44(1): 144-150
- Journal of Nanjing Agricultural University, 2021, 44(1): 144-150.
- http://dx.doi.org/10.7685/jnau.201912041
-
文章历史
- 收稿日期: 2019-12-23
在畜牧养殖中, 大量使用或滥用抗生素以提高养殖效应所带来的危害已为人们所熟知, 而其在环境中的蓄积与食品中的残留已给人类健康带来了严重危害。为了人类自身安全的考虑, 无抗养殖势在必行。我国2020年1月1日起饲料中全面禁止添加抗生素。因此, 研究和开发安全、绿色、环保型饲料添加剂已成为畜牧工作者的重要任务。益生菌是存在于自然界和动物体内的有益微生物, 因其在平衡肠道微生态中的有益作用而被广泛应用于饲料、农业、医药保健和食品等领域。联合国粮食及农业组织(FAO)和世界卫生组织(WHO)将益生菌定义为“活性微生物, 当宿主摄入了足够的量, 会对宿主的健康产生有益的影响”[1]。目前, 益生菌已越来越多地被用作抗生素的替代品[2]。
在家禽养殖中, 益生菌已显示出积极的生物学效应, 包括增加肉鸡平均日增重、提高饲料转化率、促进肠道发育以及降低肠道中有害菌的数量[3-4]。研究报道, 日粮中添加乳酸杆菌可以显著提高肉鸡肠道菌群多样性并提高肉鸡生长性能[5]。目前大多数研究主要集中在单一益生菌对肉鸡生长性能的影响, 然而与单一菌株相比, 复合益生菌具有更全面、更容易通过消化道并在肠道中定殖的优点[6]。Talebi等[7]研究表明, 多菌株益生菌可提高肉鸡的体重并降低饲料转化率。此外, 生长早期是动物生长发育的关键“窗口期”, 肉鸡肠道早期发育和微生态状况对其后期生长起决定性作用。然而, 早期添加益生菌是否促进家禽肠道内有益菌的定殖、对肠上皮发育是否发挥积极影响尚未见报道。本研究以青脚麻鸡为研究对象, 在出雏至14日龄间的饮水中添加复合益生菌以观测其对肠道细菌组成和肠上皮发育的影响。
1 材料与方法 1.1 试验动物与试验设计将144只1日龄健康青脚麻鸡随机分为3组, 分别为对照组(control, CON)、低剂量(50 mg·d-1)益生菌组(low-dose probiotic, LP)和高剂量(100 mg·d-1)益生菌组(high-dose probiotic, HP), 每组6个重复, 每个重复8只鸡。在饮水中添加益生菌, 连续饲喂2周。试验期间记录采食量和体重, 在14日龄时每组随机选取6只健康肉鸡, 每个重复随机选取1只, 断颈处死, 采集十二指肠、空肠和回肠黏膜上皮组织, 快速置于液氮中速冻, 后移入-80 ℃保存备测; 取盲肠内容物并储存于-20 ℃备测; 小肠各段组织样品放入4%甲醛缓冲溶液中固定以待制成切片。
1.2 饲养管理试验在南京市青龙山鸡场进行。雏鸡自由饮水、采食。日粮原料组成和营养组成及水平如表 1所示。
| 组成Component | 水平/% Level |
| 原料组成Ingredient component | |
| 玉米corn | 57.61 |
| 豆粕Soybean meal | 31.00 |
| 玉米蛋白粉Corn protein powder | 3.29 |
| 豆油Soybean oil | 3.11 |
| 石粉Limestone | 1.20 |
| 磷酸氢钙Dicalcium P | 2.00 |
| L-赖氨酸L-lysine | 0.34 |
| DL-蛋氨酸DL-methionine | 0.15 |
| 食盐NaCl | 0.30 |
| 预混料1 Premix1 | 1.00 |
| 营养组成2 Nutrient component | |
| 消化能3 Digestible energy | 12.56 |
| 粗蛋白Crude protein | 21.10 |
| 钙Calcium | 1.00 |
| 有效磷Available phosphorus | 0.46 |
| 赖氨酸Lysine | 1.20 |
| 蛋氨酸Methionine | 0.50 |
| 蛋氨酸+胱氨酸Methionine+cystine | 0.85 |
| 注:1)预混料为每千克日粮提供:维生素A 10 000 IU, 维生素D3 3 000 IU, 维生素E 30 IU, 维生素K3 1.3 mg, 维生素B1 2.2 mg, 维生素B2 8 mg, 烟酸40 mg, 泛酸钙10 mg, 维生素B6 4 mg, 叶酸1 mg, 生物素0.04 mg, 维生素B12 0.013 mg, 氯化胆碱600 mg, 铁80 mg, 锌60 mg, 锰110 mg, 铜8.0 mg, 碘1.1 mg, 硒0.3 mg; 2)营养组成水平为计算值; 3)消化能单位为MJ·kg-1。 Note:1)The premix provided following nutrients per kg of the diet:vitamin A 10 000 IU, vitamin D3 3 000 IU, vitamin E 30 IU, vitamin K3 1.3 mg, vitamin B1 2.2 mg, vitamin B2 8 mg, niacin 40 mg, calcium pantothenate 10 mg, vitamin B6 4 mg, folic acid 1 mg, biotin 0.04 mg, vitamin B12 0.013 mg, choline chloride 600 mg, Fe 80 mg, Zn 60 mg, Mn 110 mg, Cu 8.0 mg, I 1.1 mg, Se 0.3 mg; 2)Nutrient levels were calculated values; 3)The unit of digestible energy is MJ·kg-1. | |
试验用复合益生菌为双歧杆菌、乳酸杆菌、粪链球菌和酵母菌复合活菌制剂(江苏恒丰强生物技术有限公司)。制剂中的双歧杆菌和乳酸杆菌均不少于1.0×107 CFU·g-1, 贝氏酵母菌和粪链球菌均不少于1.0×106 CFU·g-1。
1.4 测定指标和方法 1.4.1 生长性能测定记录雏鸡1、7及14日龄体重与采食量并计算料重比。
1.4.2 雏鸡小肠绒毛高度和隐窝深度的测定将十二指肠、空肠和回肠上皮组织样本进行脱水、透明、浸蜡、包埋、修块制作石蜡切片, 石蜡切片进行苏木精-伊红(hematoxylin-eosin staining, HE)染色。每组3个样本, 每个样本连续切片2张, 每张切片选取3个不同视野, 采用Image-pro plus测量绒毛高度和隐窝深度并计算其比值。
1.4.3 实时荧光定量PCR(RT-qPCR)检测基因表达用Trizol(Invitrogen, USA)试剂提取各段小肠黏膜上皮组织中总RNA, 反转录后进行RT-qPCR, 检测各段肠道相关基因mRNA的表达, 主要包括闭合蛋白基因(occludin gene, OCLN)、闭锁小带蛋白1基因(zonula occludens-1 gene, ZO1)、紧密连接蛋白蛋白(claudin 1 gene, CLDN1)、黏蛋白2基因(mucin 2 gene, MUC2)。PCR条件:95 ℃ 3 min; 95 ℃ 30 s, 64 ℃ 20 s, 72 ℃ 20 s, 共40个循环。各基因相对表达量计算采用2-ΔΔCT法。引物由北京擎科生物工程有限公司合成。引物序列见表 2。
| 基因 Gene |
基因序列号 GenBank accession No. |
引物对序列 Primer pairs sequence(5′→3′) |
片段长度/bp Fragment size |
| OCLN | NM_205128.1 | CGCAGATGTCCAGCGGTTAC/CGAAGAAGCAGATGAGGCAGA | 177 |
| ZO-1 | NM_001030962 | CCTGGAAAGTGATGAATGTGA/TTGGTGCAAGGATTGGTGTA | 165 |
| CLDN1 | NM_001013611.2 | ATGACCAGGTGAAGAAGATGC/TGCCCAGCCAATGAAGAG | 182 |
| MUC2 | NM_001318434.1 | TCCCTCAAACAAGACCTA/AGAAGTACCACAGCGAAG | 85 |
| β-actin | L08165.1 | CCCTGTATGCCTCTGGTC/CTCGGCTGTGGTGGTGAA | 194 |
| 注:OCLN:闭合蛋白基因Occludin gene; ZO1:闭锁小带蛋白1基因Zonula occludens-1 gene; CLDN1:紧密连接蛋白基因Claudin 1 gene; MUC2:黏蛋白2基因Mucin 2 gene. | |||
采用SDS法提取样本基因组的DNA, 再进行PCR扩增和产物的混样和纯化, 使用Ion S5TMXL上机测序。将得到的原始数据(raw reads)进行拼接、过滤得到最终的有效数据(clean reads)。利用Uparse软件[8]对所有样品的全部有效数据进行聚类, 以97%的一致性将序列聚类成为操作分类单元(operational taxonomic units, OTU), 对OTU序列进行物种注释, 用Mothur方法与SILVA132的SSUrRNA数据库进行物种注释分析[9], 获得分类学信息并分别在界(kingdom)、门(phylum)、纲(class)、目(order)、科(family)、属(genus)、种(species)分类水平统计各样本的群落组成。用MUSCLE[10]软件进行快速多序列比对, 得到所有OTU序列的系统发生关系。最后对样品中数据量最少的进行均一化处理, 后续的Alpha多样性分析和Beta多样性分析都是基于均一化处理后的数据。
1.5 数据的统计分析试验数据采用SPSS 19.0进行单因素方差分析(One-way ANOVA, LSD法), 数据结果以平均值±标准误(x±SE)表示。差异显著性比较采用多重比较法。
2 结果与分析 2.1 益生菌对肉鸡生长性能的影响由表 3可知:与对照组相比, HP组肉鸡体重较高, 14日龄时体重增加约7%, 但无统计学差异(P>0.05);1~7日龄间肉鸡平均日采食量显著下降(P<0.05), 料重比极显著降低(P<0.01)。与CON相比, LP组肉鸡以上指标未见明显差异(P>0.05)。
| 项目Items | CON | LP | HP |
| 出雏体重/gInitial body weight | 37.18±0.38 | 37.35±0.52 | 37.04±0.19 |
| 7日龄体重/gBody weight at 7 days of age | 82.73±2.25 | 86.20±2.24 | 88.00±2.68 |
| 14日龄体重/gBody weight at 14 days of age | 182.74±7.51 | 179.33±7.10 | 195.09±5.50 |
| 1~7 d平均日采食量/(g·d-1) 1-7 d ADFI | 15.79±1.46a | 14.81±0.92ab | 12.42±0.26b |
| 7~14 d平均日采食量/(g·d-1) 7-14 d ADFI | 18.82±1.00ab | 17.21±1.39b | 22.10±1.15a |
| 1~7 d料重比1-7 d F/G | 2.42±0.15A | 2.16±0.21AB | 1.73±0.10B |
| 1~14 d料重比1-14 d F/G | 1.17±0.06 | 1.04±0.06 | 1.25±0.08 |
| 注:1)CON:对照组; LP:低剂量益生菌组; HP:高剂量益生菌组。2)F/G:料重比; ADFI:平均日采食量。3)不同大、小写字母表示不同处理间差异极显著(P<0.01)和显著(P<0.05)。下同。 Note:1)CON:Control group; LP:Low-dose probiotic group; HP:High-dose probiotic group. 2)F/G:Ratio of feed to gain; ADFI:Average daily feed intake. 3)Different uppercase and lowercase letters indicate that the differences between different treatments are extremely significant(P<0.01)and significant(P<0.05). The same as follows. | |||
由表 4可知:与CON相比, HP组肉鸡十二指肠绒毛长度增加(P=0.063), 隐窝变浅(P<0.05), 绒毛高度与隐窝深度的比值(V/C)极显著升高(P<0.01);LP组隐窝变浅(P<0.05)。与CON相比, HP组空肠和回肠绒毛高度极显著增加(P<0.01), 回肠V/C值显著升高(P<0.05);LP组回肠隐窝深度降低(P>0.05)。
| 项目Items | CON | LP | HP |
| 十二指肠Duodenum | |||
| 绒毛高度/μmVillus height(V) | 1 437.03±54.65ab | 1 391.85±44.90b | 1 565.36±38.83a |
| 隐窝深度/μmCrypt depth(C) | 190.82±8.39a | 164.45±4.87b | 165.31±10.77b |
| 绒毛高度/隐窝深度V/C | 7.79±0.45B | 8.81±0.47AB | 9.86±0.50A |
| 空肠Jejunum | |||
| 绒毛高度/μmVillus height | 1 036.87±49.21B | 944.89±22.14BC | 1 223.14±57.09A |
| 隐窝深度/μmCrypt depth | 190.19±14.78 | 168.10±14.22 | 178.72±9.69 |
| 绒毛高度/隐窝深度V/C | 5.94±0.69 | 6.27±0.60 | 7.33±0.62 |
| 回肠Ileum | |||
| 绒毛高度/μmVillus height | 594.76±13.14C | 708.34±16.93B | 857.24±29.60A |
| 隐窝深度/μmCrypt depth | 124.75±9.55a | 100.66±4.21b | 143.94±11.14a |
| 绒毛高度/隐窝深度V/C | 5.06±0.46Bb | 7.17±0.44Aa | 6.33±0.38ABa |
由图 1可知:与CON相比, HP组回肠ZO1和OCLN mRNA表达水平显著上调, 盲肠黏膜中ZO1和CLDN1 mRNA表达水平显著升高(P<0.05);LP组盲肠黏膜中MUC2 mRNA表达水平显著上调(P<0.05)。与LP组相比, HP组回肠黏膜上皮ZO1和OCLN mRNA及盲肠黏膜上皮ZO1表达水平显著上调(P<0.05)。
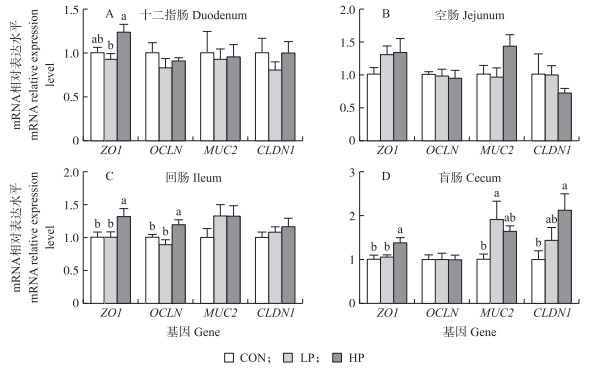
|
图 1 益生菌对肉鸡肠道黏膜上皮中紧密连接相关基因mRNA表达的影响(n=6) Fig. 1 Effects of probiotics on the mRNA expression of tight junction-related genes in intestinal mucosal epithelium of broiler chicks |
由图 2-A可知, 稀释曲线可直接反映测序数据量的合理性, 并间接反映样本中物种的丰富程度, 当样品测序数量超过40 000时, 曲线趋向饱合, 说明测序数据量渐趋合理, 更多的数据量只会产生少量新的物种(OTU)。各组盲肠菌群在门水平主要以厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)和拟杆菌门(Bacteroidetes)为主, 与CON组相比, HP组中厚壁菌门的相对丰度下降, 而变形菌门和拟杆菌门的相对丰度增加(图 2-B)。在属水平上, 盲肠内容物中菌群主要以Faecalibacterium、Alistipes和Lactobacillus等属为主(图 2-C)。与对照组相比, LP组肉鸡盲肠内容物菌群中Eisenbergiella属的丰度显著降低(P<0.05), 而HP组肉鸡盲肠内容物菌群中Sellimonas和Intestinimonas属的丰度显著增加(P<0.05)(表 5)。Metastat分析结果显示, 与对照组相比, HP组肉鸡盲肠内容物菌群的Akkermansiaceae科的丰度极显著增加(P<0.01)(图 2-D)。由表 6可见:3组的Alpha多样性指数覆盖率接近1.00, 表明每组的数据可以反映真实的盲肠微生物群水平。从observed_species和PD_whole_tree指数来看, HP组中盲肠微生物菌群的丰度分别比对照组高14.7%和13.8%, 但3组间Shannon、Simpson、Chao1和ACE指数无显著差异。
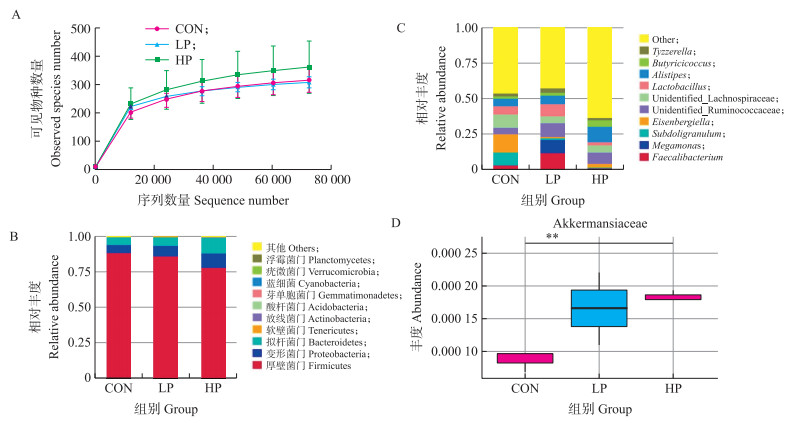
|
图 2 益生菌对肉鸡盲肠内容物微生物菌群的影响 Fig. 2 Effects of probiotics on microflora of cecal contents in broiler chicks A.稀释曲线; B.门水平盲肠内容物物种相对丰度; C.属水平盲肠内容物物种相对丰度; D.基于Metastat科水平物种的多样性分析。**表示HP组与CON组相比差异极显著(P<0.01)。 A. Rarefaction curve; B. Relative abundance of species at the phylum level; C:Relative abundance of species at the genus level; D:Analysis of the species diversity at family level based on Metastat. ** means the difference between HP group and CON group is significant at the 0.01 level. |
| 菌属 | CON | LP | HP |
| Faecalibacterium | 0.032±0.032 | 0.119±0.112 | 0.011±0.006 |
| Eisenbergiella | 0.126±0.056a | 0.010±0.003b | 0.024±0.017ab |
| Lactobacillus | 0.059±0.055 | 0.086±0.044 | 0.024±0.012 |
| Intestinimonas | 0.000±0.000b | 0.003±0.001a | 0.002±0.001ab |
| Sellimonas | 0.004±0.001b | 0.014±0.003a | 0.007±0.004ab |
| Others | 0.466±0.048 | 0.430±0.109 | 0.636±0.037 |
| 项目Items | 对照组CON | 低剂量益生菌组LP | 高剂量益生菌组HP |
| Observed_species | 313.67±30.91 | 306.00±13.87 | 359.67±64.83 |
| 香农指数Shannon index | 5.05±0.09 | 5.11±0.43 | 5.34±0.45 |
| 辛普森指数Simpson index | 0.93±0.01 | 0.92±0.04 | 0.94±0.02 |
| Chao1指数Chao1 index | 336.05±28.02 | 333.87±21.91 | 433.80±107.10 |
| ACE指数ACE index | 343.92±30.46 | 334.98±20.77 | 413.84±81.67 |
| 覆盖率Goods_coverage | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| 谱系多样性PD_whole_tree | 21.36±2.81 | 18.73±0.98 | 24.31±4.89 |
益生菌可以改善肠道黏膜屏障功能从而提高肉鸡的生长性能[11]。枯草芽胞杆菌fmbJ能显著提高肉鸡饲料转化率(FCR)[12]。但也有研究报道, 益生菌对肉鸡FCR影响很小或没有影响[13]。本试验结果显示, 添加高剂量益生菌能显著降低1~7日龄肉鸡的料重比。以上研究结果的不一致性可能与益生菌的菌株、剂量、添加起止时间及饲料成分等因素有关[14]。
小肠上皮结构是营养物质被消化和吸收的基础, 而绒毛高度、隐窝深度及其比值是决定物质消化和吸收的关键因素。绒毛高度增加可以扩大营养物质的消化吸收面积, 提高小肠的吸收能力[15]。研究发现采食益生菌后可引起小肠结构的显著变化, 如Samanya等[16]发现纳豆枯草芽胞杆菌显著增加小肠的绒毛高度, Song等[17]也得到了类似的结果。本试验结果也显示益生菌对肠道绒毛高度、隐窝深度及其比值有积极影响。与LP组相比, HP组肉鸡小肠绒毛高度显著增加, 这提示高剂量益生菌效果优于低剂量益生菌。肠上皮细胞是一层动态的细胞层并作为肠腔内容物和免疫系统之间的屏障。该屏障的功能是过滤一些促炎因子, 如病原体、毒素和抗原。肠上皮细胞发挥这种功能依赖于肠道的紧密连接(TJ)和黏附连接(AJ)[18]。黏液层提供了抵御物理和化学损伤以及肠道病原体入侵的第一道防线。MUC2是防止病原微生物到达黏膜层的基础[19]。Smirnov等[20]研究表明益生菌处理显著增加肉鸡肠道中的MUC2 mRNA表达。Peng等[21]报道B.subtilis CW14通过提高ZO1蛋白表达从而减轻紧密连接损伤。Gadde等[22]研究表明益生菌组促使肉仔鸡回肠的OCLN、ZO1 mRNA表达升高。本试验结果显示, 添加高剂量益生菌能显著增加回肠ZO1和OCLN mRNA表达水平及盲肠黏膜中ZO1和CLDN1 mRNA表达水平。与低剂量组相比, 饲喂高剂量益生菌可显著上调十二指肠、回肠和盲肠上皮中ZO1的基因表达。这也提示高剂量益生菌添加对肉鸡肠道屏障的作用要优于低剂量益生菌。本试验结果显示, 添加复合益生菌可上调小肠上皮紧密连接相关基因的表达, 提高肠绒毛高度, 提示其潜在地促进肠道功能及生长性能的作用。
在门水平上, 肉鸡肠道优势菌主要是拟杆菌门和厚壁菌门。在健康的肠道环境中, 优势菌群起主导作用, 并且能抵抗外来细菌的定殖。本试验结果显示低剂量益生菌处理显著增加了Sellimonas和Intestini-monas属的丰度。Intestinimonas是一种产短链脂肪酸(short chain fatty acid, SCFA)的细菌[23], 且SCFA对维持肠道健康具有重要作用, 如作为营养素和能量源来保护肠黏膜[24]。LP组中Sellimonas属水平也显著增加, 但Sellimonas的功能尚未见报道[25]。在本试验中, Eisenbergiella的丰度在LP组中显著下降, 虽然该属的生物学功能信息非常有限, 但是Eisenbergiella属于Lachnospiraceae, 有研究表明Lachnospiraceae的增加可能与代谢性疾病如糖尿病的发生有关[26], 这提示Eisenbergiella丰度的下降可能对机体有益。Akkermansiaceae为疣微菌门的第Ⅱ科(Family Ⅱ. Akkermansiaceae fam. nov.), Akkermansia是科内唯一菌属[27]。研究表明A.muciniphila的丰度与小鼠和人类肥胖以及Ⅱ型糖尿病等的发病率呈负相关[28]。肠道炎症疾病患者的A.muciniphila丰度显著低于普通人。Vemuri等[29]报道嗜酸乳杆菌DDS-1处理增加有益细菌如Akkermansia spp.的丰度, 我们的结果与之一致。A.muciniphila定殖在黏液层[30], 本试验中MUC2 mRNA表达的增加与盲肠中Akkermansiaceae丰度的增加相一致。Intestinimonas和Akkermansiaceae丰度的增加以及Eisenbergiella丰度的减少意味着益生菌可能改变盲肠微生物菌群的组成, 益生菌处理可能会增加有益细菌的定殖并减少有害细菌的定殖。我们使用Observed_species、Shannon、Simpson、Chao1、ACE、goods_coverage和PD_whole_tree来测量样本中微生物的丰度和多样性, 尽管这些指标没有显着差异, 但在高剂量益生菌处理组其数值都升高, 提示饲喂益生菌有增加肠道菌群多样性的潜在作用。
早期添加复合益生菌可以增加肉仔鸡盲肠内有益菌的定殖并减少有害菌的定殖, 改善雏鸡肠道结构并促进小肠发育, 增强肠道间的紧密连接, 改善早期生长性能并对后期生长具有潜在的益处, 且添加高剂量益生菌的效果要优于低剂量。因此, 复合益生菌有望成为无抗养殖中绿色、安全的添加剂。
| [1] |
Afrc R F. Probiotics in man and animals[J]. The Journal of Applied Bacteriology, 1989, 66(5): 365-378. DOI:10.1111/j.1365-2672.1989.tb05105.x |
| [2] |
Ayasan T. Effects of dietary inclusion of protexin(probiotic)on hatchability of Japanese quails[J]. Indian Journal of Animal Science, 2013, 83(1): 78-81. |
| [3] |
Perić L, Milošević N, Žikić D, et al. Effects of probiotic and phytogenic products on performance, gut morphology and cecal microflora of broiler chickens[J]. Archives Animal Breeding, 2010, 53(3): 350-359. DOI:10.5194/aab-53-350-2010 |
| [4] |
Park J H, Kim I H. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks[J]. Poultry Science, 2014, 93(8): 2054-2059. DOI:10.3382/ps.2013-03818 |
| [5] |
Patterson J A, Burkholder K M. Application of prebiotics and probiotics in poultry production[J]. Poultry Science, 2003, 82(4): 627-631. DOI:10.1093/ps/82.4.627 |
| [6] |
Timmerman H M, Koning C J M, Mulder L, et al. Monostrain, multistrain and multispecies probiotics:a comparison of functionality and efficacy[J]. International Journal of Food Microbiology, 2004, 96(3): 219-233. DOI:10.1016/j.ijfoodmicro.2004.05.012 |
| [7] |
Talebi A, Amirzadeh B, Mokhtari B, et al. Effects of a multi-strain probiotic(PrimaLac)on performance and antibody responses to Newcastle disease virus and infectious bursal disease virus vaccination in broiler chickens[J]. Avian Pathology, 2008, 37(5): 509-512. DOI:10.1080/03079450802356995 |
| [8] |
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads[J]. EMBnet Journal, 2011, 17(1): 10. DOI:10.14806/ej.17.1.200 |
| [9] |
Haas B J, Gevers D, Earl A M, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons[J]. Genome Research, 2011, 21(3): 494-504. DOI:10.1101/gr.112730.110 |
| [10] |
Edgar R C. UPARSE:highly accurate OTU sequences from microbial amplicon reads[J]. Nature Methods, 2013, 10(10): 996. DOI:10.1038/nmeth.2604 |
| [11] |
唐志刚, 王俊峰, 温超, 等. 益生菌对肉鸡生产性能、免疫器官指数和血清指标的影响[J]. 江苏农业科学, 2010, 38(4): 208-210. Tang Z G, Wang J F, Wen C, et al. Effects of probiotics on performance, immune organ index and serum index of broilers[J]. Jiangsu Agricultural Science, 2010, 38(4): 208-210 (in Chinese with English abstract). DOI:10.3969/j.issn.1002-1302.2010.04.085 |
| [12] |
Bai K W, Huang Q, Zhang J F, et al. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens[J]. Poultry Science, 2016, 96(1): 74-82. |
| [13] |
Zhang Z F, Zhou T X, Ao X, et al. Effects of β-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize-soybean meal based diets[J]. Livestock Science, 2012, 150(1/2/3): 419-424. |
| [14] |
Lee K, Lillehoj H S, Siragusa G R. Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens[J]. The Journal of Poultry Science, 2010, 47(2): 106-114. DOI:10.2141/jpsa.009096 |
| [15] |
Caspary W F. Physiology and pathophysiology of intestinal absorption[J]. The American Journal of Clinical Nutrition, 1992, 55(1): 299S-308S. DOI:10.1093/ajcn/55.1.299s |
| [16] |
Samanya M, Yamauchi K. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto[J]. Comp Biochem Physiol A Mol Integr Physiol, 2002, 133(1): 95-104. DOI:10.1016/S1095-6433(02)00121-6 |
| [17] |
Song J, Xiao K, Ke Y L, et al. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress[J]. Poultry Science, 2014, 93(3): 581-588. DOI:10.3382/ps.2013-03455 |
| [18] |
Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions[J]. Nature Reviews Molecular Cell Biology, 2001, 2(4): 285-293. DOI:10.1038/35067088 |
| [19] |
Johansson M E V, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria[J]. Proc Natl Acad Sci USA, 2008, 105(39): 15064-15069. DOI:10.1073/pnas.0803124105 |
| [20] |
Smirnov A, Perez R, Amit-Romach E, et al. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation[J]. The Journal of Nutrition, 2005, 135(2): 187-192. DOI:10.1093/jn/135.2.187 |
| [21] |
Peng M X, Liu J W, Liang Z H. Probiotic Bacillus subtilis CW14 reduces disruption of the epithelial barrier and toxicity of ochratoxin A to Caco-2 cells[J]. Food and Chemical Toxicology, 2019, 126: 25-33. DOI:10.1016/j.fct.2019.02.009 |
| [22] |
Gadde U, Oh S T, Lee Y S, et al. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens[J]. Probiotics and Antimicrobial Proteins, 2017, 9(4): 397-405. DOI:10.1007/s12602-017-9275-9 |
| [23] |
Li L, Guo W L, Zhang W, et al. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats[J]. Food & Function, 2019, 10(5): 2560-2572. |
| [24] |
den Besten G, van Eunen K, Groen A K, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. Journal of Lipid Research, 2013, 54(9): 2325-2340. DOI:10.1194/jlr.R036012 |
| [25] |
Seo B, Yoo J E, Lee Y M, et al. Sellimonas intestinalis gen. nov., sp. nov., isolated from human faeces[J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66(2): 951-956. DOI:10.1099/ijsem.0.000817 |
| [26] |
Kameyama K, Itoh K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice[J]. Microbes and Environments, 2014, 29(4): 427-430. DOI:10.1264/jsme2.ME14054 |
| [27] |
Derrien M, Belzer C, de Vos W M. Akkermansia muciniphila and its role in regulating host functions[J]. Microbial Pathogenesis, 2017, 106: 171-181. DOI:10.1016/j.micpath.2016.02.005 |
| [28] |
Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity[J]. Proc Natl Acad Sci USA, 2013, 110(22): 9066-9071. DOI:10.1073/pnas.1219451110 |
| [29] |
Vemuri R, Gundamaraju R, Shinde T, et al. Lactobacillus acidophilus DDS-1 modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice[J]. Nutrients, 2019, 11(6): 1297. DOI:10.3390/nu11061297 |
| [30] |
Zhang T, Li Q Q, Cheng L, et al. Akkermansia muciniphila is a promising probiotic[J]. Microbial Biotechnology, 2019, 12(6): 1109-1125. DOI:10.1111/1751-7915.13410 |




