文章信息
- 公茂勇, 王燕, 徐良, 王娟娟, 谢洋, 范莲雪, 陈微, 柳李旺
- GONG Maoyong, WANG Yan, XU Liang, WANG Juanjuan, XIE Yang, FAN Lianxue, CHEN Wei, LIU Liwang
- 不同种质萝卜肉质根硫苷组分及含量分析
- The composition and content of glucosinolate in different radish (Raphanus sativus L.)genotypes
- 南京农业大学学报, 2019, 42(3): 413-420
- Journal of Nanjing Agricultural University, 2019, 42(3): 413-420.
- http://dx.doi.org/10.7685/jnau.201809014
-
文章历史
- 收稿日期: 2018-09-07
2. 全国农业技术推广服务中心, 北京 100125
2. The National Agro-Tech Extension and Service Center, Beijing 100125, China
硫苷是一类广泛存在于十字花科植物中的重要次级代谢产物[1]。硫苷及其降解产物在植物风味、营养价值、防御体制以及人类健康等方面具有重要作用[2-5]。关于硫苷组分及其降解产物的生物活性及功能方面研究广受关注。迄今为止, 已从十字花科植物中鉴定出130多种硫苷组分[6]。根据前体氨基酸来源的不同, 硫苷通常分为3类:1)脂肪族硫苷, 侧链来源于蛋氨酸、异亮氨酸、亮氨酸或缬氨酸; 2)芳香族硫苷, 来源于苯丙氨酸或酪氨酸; 3)吲哚族硫苷, 来源于色氨酸[5, 7-8]。
硫苷是亲水性的稳定化合物, 通常存在于植物组织液泡中[9]; 黑芥子酶主要分布于黑芥子酶异细胞(myrosin idioblasts, MI)中[10-11]。当植物组织细胞受损伤时, 硫苷与黑芥子酶接触, 硫苷在黑芥子酶的催化作用下水解, 产生不稳定的葡糖苷配基和葡萄糖。这些不稳定的中间产物, 由于植物物种、硫苷侧链结构及反应条件等差异, 形成一系列具有生物活性的化合物, 例如异硫代氰酸盐、硫代氰酸盐和乙腈等。
目前植物硫苷的提取主要采用硫酸酯酶脱硫法, 可有效降低硫苷极性[12]。硫苷检测方法有很多, 如气质色谱法、紫外光谱法、比色法、毛细管电泳法、荧光分析法等, 但普遍使用的是高效液相色谱法(HPLC)[13-14], 再结合质谱法(MS)对未知硫苷进行结构鉴定[15-17]。
在模式植物拟南芥和其他十字花科作物中有关硫苷的研究较多[18-20], 但对于萝卜肉质根中硫苷的报道相对较少, 且主要侧重于硫苷含量和组分检测分析[21-24]。开展不同种质作物中硫苷组分和含量研究可揭示种内变异, 对于分析基因型、硫苷与抗性等相关关系具有重要作用。因此, 本试验通过LC-MS和HPLC测定萝卜肉质根中硫苷的组分和含量, 确定萝卜种质对总硫苷和单个硫苷含量的影响, 并通过主成分分析(PCA)研究总硫苷与单个硫苷含量之间的相关性, 为有效提高萝卜肉质根品质性状遗传改良效率、培育优良萝卜新品种提供理论基础。
1 材料与方法 1.1 试验材料试验共选取了44份具有代表性的不同根型(长圆柱、短圆柱、长圆锥、短圆锥、扁圆、近圆、纺锤形等)、不同皮色(红、白、青、紫、黑等)以及适于鲜食和生食等特征特性的萝卜品种。2016年9月将不同种质萝卜种子播种于南京农业大学江浦园艺试验站, 设置3个重复, 每个重复6个单株, 正常水肥管理。于11月选取成熟期长势良好且均一的肉质根, 切薄片后液氮速冻, 于液氮中粉碎后真空冷冻干燥8 h, 于超低温冰箱中保存备用。
1.2 萝卜中硫苷含量的测定硫苷含量提取参照杨丽娟等[22]和Ishida等[25]的方法并略作修改。称取0.2 g样品于10 mL离心管中, 75 ℃恒温水浴锅中预热1 min后加入4 mL 80%(体积分数)甲醇溶液和100 μL 2-丙烯基硫苷(内标物), 然后75 ℃水浴15 min, 期间每2~3 min涡旋1次, 取出离心管并放置在冰上快速冷却后, 再加入1 mL 0.4 mol · mL-1醋酸钡溶液, 涡旋15 s, 4 500 r · min-1离心10 min, 转移上清液于新10 mL离心管, 沉淀再用80%甲醇溶液浸提2次; 合并上清液定容至10 mL得到样品液。取3 mL样品液缓慢流经醋酸型DEAE SephadexTM A25柱, 然后用2 mL 20 μmol · L-1醋酸钠溶液冲洗层析柱; 待液体排干后加入150 μL稀释的硫酸酯酶溶液(Type H-1, Sigma), 37 ℃酶解16 h后用2.5 mL超纯水洗脱, 0.45 μm水系滤膜过滤后收集滤液于2.0 mL试管中, -20 ℃保存备用。
1.3 HPLC检测采用高效液相色谱仪(Waters 2489 UV检测器, 1525双线性梯度泵, C18柱)检测硫苷组分和含量。流动相A为超纯水, 流动相B为色谱级乙腈, 柱温30 ℃, 检测波长229 nm, 进样量20 μL; 流速1 mL · min-1。洗脱梯度:0~7 min, B 3%;8~32 min, B 3%→20%;33~38 min, B 20%;39~42 min, B 20%→100%;43~45 min, B 100%;46~51 min, B 100%→3%。
以2-丙烯基硫苷为内标, 根据保留时间、峰面积和质荷比(m/z)值对脱硫硫苷组分进行定性、定量测定。定量公式为mo=(Ai/As)×F×Ns/m。式中:mo为脱硫硫苷含量(μmol · g-1); Ai为硫苷组分峰面积; As为内标峰面积; F为相对响应因子; Ns为内标含量(μmol); m为冻干样品质量(g)。
1.4 LC-MS检测液质联用(LC-MS)质量分析器:LTQ, FT Orbitrap; 质荷比(m/z):100~600;鞘气压力:35 arb; 毛细管温度:300 ℃; 分辨率:30 000;喷雾电压:4.0 kV; 管状透镜电压:90 V; 扫描模式:正离子; 加热器温度:100 ℃; 辅助气压力:10 arb; 离子源:ESI; 毛细管电压:28 V; 进样量:5 μL; 流速:0.5 mL · min-1。根据质谱图中重要特征碎片离子对硫苷进行定性[26]。
1.5 数据分析数据采用SPSS 19.0软件进行方差分析、差异显著性分析与主成分分析。
2 结果与分析 2.1 萝卜肉质根中硫苷的定性分析采用HPLC法在44份萝卜种质肉质根硫苷提取物中共检测到12个色谱峰(图 1)。为鉴定相应的硫苷组分, 通过LC-MS检测对应色谱峰的质荷比(m/z)值。硫苷经过硫酸酯酶水解脱去1个硫酸根, 形成的脱硫硫苷在质谱仪电喷雾碰撞激活条件下失去1个葡萄糖分子(G)(m/z=162.1), 产生脱硫硫苷MS重要特征碎片离子[M-G+H]+, 还会产生[M+H]+、[M+Na]+、[M+K]+中的1或2个, 由此可计算脱硫硫苷的相对分子质量, 从而对硫苷分子进行定性分析。由表 1可见:共检测到12种硫苷, 其中8种脂肪族硫苷(GRE、n-Heptyl、GIV、GRH、GER、PRO、RAA、GBN), 4种吲哚族硫苷(4OH、GBC、4ME、NEO)。
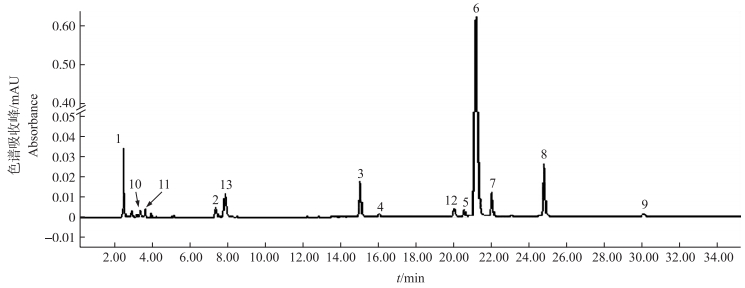
|
图 1 萝卜肉质根脱硫硫苷的HPLC色谱图(种质14-B24) Fig. 1 HPLC chromatogram of desulphglucosinolates in radish roots(genotype 14-B24) |
| 编号 No. |
化学名 Chemical name |
名称及简称 Trivial name and abbreviation |
脱硫相对分子质量 Desulfurization relative molecular mass |
m/z值 m/z value |
| 1 | 4-甲基亚磺酰基-3-丁烯基硫苷 | Glucoraphenin(GRE) | 355 | 356[M+H]+, 378[M+Na]+, 194[M-G+H]+ |
| 2 | 庚基硫苷 | n-Heptyl | 337 | 338[M+H]+, 176[M-G+H]+ |
| 3 | 4-羟基吲哚-3-甲基硫苷 | 4-hydroxyglucobrassicin(4OH) | 384 | 223[M+H]+, 385[M-G+H]+ |
| 4 | 4-甲基硫代丙基硫苷 | Glucoiberverin(GIV) | 327 | 328[M+H]+, 380[M+Na]+, 166[M-G+H]+ |
| 5 | 4-甲硫基丁基硫苷 | Glucoerucin(GER) | 341 | 342[M+H]+, 364[M+Na]+, 180[M-G+H]+ |
| 6 | 4-甲硫基-3-丁烯基硫苷 | Glucoraphasatin(GRH) | 339 | 340[M+H]+, 178[M-G+H]+ |
| 7 | 吲哚-3-甲基硫苷 | Glucobrassicin(GBC) | 368 | 369[M+H]+, 207[M-G+H]+ |
| 8 | 4-甲氧基吲哚-3-甲基硫苷 | 4-methoxyglucobrassicin(4ME) | 398 | 399[M+H]+, 237[M-G+H]+ |
| 9 | 1-甲氧基吲哚-3-甲基硫苷 | Neoglcobrassincin(NEO) | 398 | 399[M+H]+, 237[M-G+H]+ |
| 10 | 2-羟基-3-丁烯基硫苷 | Progoitrin(PRO) | 309 | — |
| 11 | 4-甲基亚磺酰丁基硫苷 | Glucoraphanin(RAA) | 357 | — |
| 12 | 4-戊烯基硫苷 | Glucobrassicanapin(GBN) | 307 | — |
| 13 | 2-丙烯基硫苷(内标) | Sinigrin(SIN) | 279 |
由表 2和图 2可知:不同种质萝卜肉质根中硫苷含量差异明显。44份萝卜种质中总硫苷含量最高的为基因型13-6112(23.23 μmol · g-1), 其次为基因型13-6166(22.55 μmol · g-1), 而含量最低的为14-B24(1.61 μmol · g-1), 平均含量为10.73 μmol · g-1。硫苷总量占干质量的比例变化区间为0.14%~0.97%。
| μmol · g-1 | ||||||
| 基因型Genotype | GRE | PRO | RAA | n-Heptyl | GIV | 4OH |
| 13-6136 | 0.410±0.075cde | 0.095±0.006ijkl | 0.072±0.009klmn | 0.291±0.163def | 0.188±0.077efg | — |
| 13-6166 | 0.164±0.018jklm | 0.258±0.048d | 0.187±0.018def | 0.140±0.095hijk | 0.252±0.104cd | — |
| 13-6103 | 0.299±0.073efgh | 0.080±0.094jklm | 0.069±0.081klmn | 0.128±0.048klm | 0.513±0.069a | 0.013±0.013klm |
| 13-6102 | 0.120±0.008lmno | 0.402±0.005b | 0.317±0.032b | 0.562±0.136b | 0.115±0.068hij | — |
| 13-6145 | 0.474±0.231cd | 0.206±0.061def | 0.121±0.040ghij | 0.395±0.102bcd | 0.371±0.041b | — |
| 13-6122 | 0.192±0.018ghij | 0.515±0.074a | 0.374±0.032a | 0.834±0.202a | 0.147±0.040ghi | 0.093±0.093a |
| 13-6099 | 0.236±0.020ghi | 0.167±0.014fgh | 0.132±0.011ghi | 0.269±0.051def | 0.237±0.063cde | 0.041±0.041def |
| 13-6082 | 0.172±0.024ijklm | 0.159±0.001fgh | 0.124±0.010ghij | 0.348±0.112cde | 0.144±0.037ghi | — |
| 13-6093 | 0.176±0.041ijk | 0.235±0.101de | 0.194±0.085de | 0.298±0.039def | 0.211±0.025def | — |
| 13-6160 | 0.136±0.020lmno | 0.184±0.044efg | 0.151±0.036efgh | 0.228±0.133efg | 0.174±0.021fgh | — |
| 13-6095 | 0.418±0.077cde | 0.136±0.020ghi | 0.111±0.012ghijk | 0.233±0.023efg | 0.169±0.007fgh | 0.049±0.049cd |
| 13-6163 | 0.095±0.011mnop | 0.169±0.019fgh | 0.142±0.015fgh | 0.156±0.018hijk | 0.134±0.010ghij | — |
| 13-6128 | 0.538±0.157c | 0.075±0.037jklm | 0.076±0.034jklm | 0.211±0.058fgh | 0.335±0.063b | — |
| 13-6132 | 0.207±0.051ghij | 0.329±0.014c | 0.270±0.013c | 0.323±0.071def | 0.039±0.004opq | — |
| 13-6091 | 0.235±0.034ghi | 0.336±0.023c | 0.267±0.008c | 0.294±0.059def | 0.101±0.007ijk | 0.018±0.018hijk |
| 13-6090 | 0.146±0.029lmno | 0.346±0.042bc | 0.291±0.031c | 0.239±0.165efg | 0.259±0.008cd | 0.045±0.045de |
| 13-6078 | 0.110±0.022mnop | 0.189±0.009efg | 0.159±0.005efg | 0.161±0.052ghij | 0.131±0.004hij | 0.015±0.015jkl |
| 13-6109 | 0.121±0.038mno | 0.095±0.002ijklm | 0.079±0.002jklm | 0.205±0.161ghi | 0.120±0.006hij | — |
| 13-6112 | 0.276±0.123fghi | 0.190±0.039efg | 0.148±0.027efgh | 0.344±0.069cde | 0.274±0.054c | 0.026±0.026fgh |
| 13-6108 | 0.236±0.179ghi | 0.134±0.013ghi | 0.105±0.011ijkl | 0.248±0.064def | 0.168±0.023fgh | — |
| 13-6135 | 0.162±0.063klmn | 0.383±0.021bc | 0.318±0.019b | 0.410±0.182bcd | 0.210±0.009def | — |
| 13-6081 | 0.100±0.054mnop | 0.480±0.009a | 0.398±0.008a | 0.479±0.131bcd | 0.192±0.006efg | — |
| 13-6089 | 0.254±0.214lmno | 0.338±0.067c | 0.277±0.049bc | 0.509±0.132bc | 0.218±0.037cdef | 0.030±0.030fg |
| 14-B3 | 0.437±0.027cd | 0.116±0.003hijk | 0.108±0.007hijk | 0.075±0.028lmn | 0.044±0.002mno | 0.059±0.019bc |
| 14-B5 | 1.447±0.010a | 0.053±0.010klm | 0.060±0.005lmn | 0.000±0.000s | 0.021±0.001qrs | — |
| 14-B9 | 0.344±0.027def | 0.058±0.006jklm | 0.065±0.015klmn | 0.062±0.030mno | 0.040±0.002opq | 0.034±0.001efg |
| 14-B11 | 0.064±0.006nop | 0.046±0.005m | 0.054±0.018p | 0.039±0.068nop | 0.006±0.003pqr | — |
| 14-B12 | 0.131±0.010lmno | 0.119±0.004hij | 0.114±0.006ghijk | 0.044±0.024opq | 0.138±0.008ghij | — |
| 14-B13 | 0.172±0.016ijkl | 0.060±0.010jklm | 0.056±0.010mnop | 0.078±0.044lmn | 0.052±0.003mno | — |
| 14-B18 | 0.118±0.005lmno | 0.058±0.004jklm | 0.046±0.002nop | 0.126±0.089klm | 0.023±0.001pqr | — |
| 14-B19 | 0.111±0.010lmno | 0.032±0.011lm | 0.027±0.002p | 0.108±0.081klmn | 0.004±0.001s | — |
| 14-B24 | 0.037±0.011p | 0.042±0.002lm | 0.061±0.007lmn | 0.028±0.025pqr | 0.015±0.00qrs | — |
| 14-B26 | 0.057±0.021op | 0.078±0.002jklm | 0.150±0.020efgh | 0.063±0.037lmn | 0.084±0.008jkl | — |
| 14-B44 | 0.808±0.035b | 0.061±0.017jklm | 0.044±0.021nop | 0.072±0.025lmn | 0.076±0.006klm | 0.036±0.008def |
| 14-B45 | 0.087±0.001mnop | 0.034±0.002lm | 0.032±0.001op | 0.165±0.079ghi | 0.005±0.001s | — |
| 14-B46 | 0.305±0.054efg | 0.070±0.040jklm | 0.111±0.014ghijk | 0.085±0.036lmn | 0.006±0.001s | 0.065±0.003b |
| 14-B47 | 0.218±0.011ghij | 0.095±0.012ijkl | 0.220±0.028d | 0.063±0.027lmn | 0.090±0.003ijk | — |
| 14-B50 | 0.255±0.051fghi | 0.062±0.020jklm | 0.067±0.016klmn | 0.050±0.016nop | 0.117±0.004hip | 0.029±0.001fgh |
| 14-B51 | 0.108±0.003mnop | 0.033±0.003lm | 0.059±0.022lmn | 0.062±0.041mno | 0.017±0.001qrs | — |
| 14-B53 | 0.136±0.022lmno | 0.052±0.000klm | 0.065±0.010klmn | 0.066±0.063lmn | 0.005±0.001s | — |
| 14-B54 | 0.438±0.012cd | 0.064±0.009jklm | 0.061±0.003lmn | 0.076±0.040lmn | 0.071±0.006lmn | 0.027±0.047fgh |
| 14-B59 | 0.064±0.020nop | 0.028±0.003lm | 0.026±0.006nop | 0.049±0.053opq | 0.027±0.006s | 0.003±0.006m |
| 14-B60 | 0.103±0.001mnop | 0.057±0.005jklm | 0.092±0.013ijkl | 0.051±0.033nop | 0.021±0.002pqr | — |
| 14-B61 | 0.195±0.004ghij | 0.034±0.001lm | 0.033±0.002op | 0.020±0.007rs | 0.013±0.001rs | 0.015±0.001ijkl |
| 基因型Genotype | GBN | GER | GRH | GBC | 4ME | NEO |
| 13-6136 | 0.234±0.017ghi | 0.208±0.017de | 14.349±0.670cde | 1.814±0.081a | 0.761±0.761a | 0.014±0.001c |
| 13-6166 | 0.599±0.108a | 0.239±0.108bcd | 19.659±4.229ab | 0.795±0.180c | 0.535±0.535b | 0.021±0.017c |
| 13-6103 | 0.280±0.038fgh | 0.109±0.038ijk | 9.127±1.146ijk | 0.080±0.009lmn | 0.071±0.071s | 0.006±0.007c |
| 13-6102 | 0.082±0.003pqr | 0.065±0.003op | 5.856±0.119mno | 0.800±0.006c | 0.275±0.275fgh | 0.017±0.014c |
| 13-6145 | 0.357±0.021de | 0.208±0.021de | 13.780±2.236efg | 0.330±0.186f | 0.276±0.276fgh | 0.029±0.010c |
| 13-6122 | 0.198±0.024hij | 0.206±0.024de | 10.357±0.330h | 0.341±0.014f | 0.233±0.233hij | 0.032±0.005c |
| 13-6099 | 0.299±0.023ef | 0.134±0.023ghi | 9.524±0.569i | 0.189±0.019hij | 0.243±0.243ghi | 0.006±0.001c |
| 13-6082 | 0.174±0.009jkl | 0.104±0.009jkl | 9.464±0.368ij | 0.100±0.015klmn | 0.203±0.203ijk | 0.022±0.004c |
| 13-6093 | 0.193±0.007ijk | 0.111±0.007ijk | 7.567±0.750klmn | 0.060±0.057mno | 0.137±0.137lmn | — |
| 基因型Genotype | GBN | GER | GRH | GBC | 4ME | NEO |
| 13-6160 | 0.137±0.061lmn | 0.079±0.061klm | 5.552±1.913opq | 0.059±0.034mno | 0.137±0.137lmn | 0.010±0.012c |
| 13-6095 | 0.165±0.009klm | 0.133±0.009ghi | 9.264±0.599ij | 0.117±0.015klm | 0.217±0.217hijk | 0.009±0.002c |
| 13-6163 | 0.243±0.042fgh | 0.089±0.042jkl | 7.136±0.970lmno | 0.340±0.047f | 0.338±0.338de | 0.004±0.001c |
| 13-6128 | 0.388±0.075cd | 0.191±0.075ef | 15.527±2.263bcd | 1.061±0.110b | 0.401±0.401cd | 0.077±0.010b |
| 13-6132 | 0.170±0.015jkl | 0.109±0.015ijk | 8.978±0.768ij | 0.612±0.058d | 0.080±0.080rs | 0.010±0.002c |
| 13-6091 | 0.057±0.007qr | 0.055±0.007p | 2.638±0.118st | 0.041±0.002opq | 0.082±0.082rs | 0.001±0.002c |
| 13-6090 | 0.203±0.015hij | 0.118±0.015hij | 8.116±0.924jklm | 0.044±0.011opq | 0.223±0.223hijk | 0.011±0.001c |
| 13-6078 | 0.300±0.009ef | 0.080±0.009klm | 5.654±0.221nopq | 0.104±0.011klmn | 0.226±0.226hij | 0.005±0.001c |
| 13-6109 | 0.118±0.019mno | 0.076±0.019lmn | 5.967±0.536nopq | 0.462±0.029e | 0.148±0.148klm | — |
| 13-6112 | 0.229±0.041ghi | 0.117±0.041hij | 21.246±10.111a | 0.491±0.081e | 0.185±0.185ijkl | 0.011±0.002c |
| 13-6108 | 0.102±0.017nop | 0.052±0.017p | 3.461±0.418rst | 0.148±0.017ijk | 0.113±0.113opq | — |
| 13-6135 | 0.191±0.016ijk | 0.113±0.016ijk | 8.436±0.335jkl | 0.142±0.006jkl | 0.255±0.255fgh | 0.009±0.001c |
| 13-6081 | 0.151±0.029lmn | 0.100±0.029jkl | 6.065±0.526mno | 0.162±0.013ijk | 0.369±0.369de | — |
| 13-6089 | 0.545±0.113ab | 0.213±0.113de | 16.861±2.960abc | 0.303±0.052fg | 0.269±0.269fgh | 0.021±0.004c |
| 14-B3 | 0.173±0.023ijk | 0.202±0.027e | 9.670±0.708i | 0.194±0.011hij | 0.217±0.180hijk | 0.004±0.004c |
| 14-B5 | 0.068±0.001pqr | 0.085±0.004jkl | 4.671±0.096pqr | 0.011±0.001r | 0.121±0.005opq | — |
| 14-B9 | 0.342±0.013cd | 0.250±0.007bc | 12.437±0.087h | 0.029±0.001pq | 0.072±0.003s | — |
| 14-B11 | 0.066±0.006opq | 0.074±0.009nop | 2.871±0.099stu | 0.031±0.001mno | 0.073±0.003mno | — |
| 14-B12 | 0.142±0.009klm | 0.142±0.008ghi | 7.123±0.318lmno | 0.482±0.025e | 0.295±0.017efg | — |
| 14-B13 | 0.131±0.023lmn | 0.168±0.027fg | 6.033±0.595opq | 0.144±0.029jkl | 0.119±0.100opq | 0.005±0.002c |
| 14-B18 | 0.089±0.002nop | 0.088±0.004jkl | 4.613±0.089qrs | 0.051±0.001mno | 0.186±0.003ijkl | — |
| 14-B19 | 0.069±0.001pqr | 0.073±0.006mno | 3.385±0.067st | 0.046±0.001nop | 0.106±0.001pqr | — |
| 14-B24 | 0.019±0.001r | 0.018±0.001q | 1.267±0.020u | 0.062±0.001mno | 0.065±0.002s | — |
| 14-B26 | 0.120±0.002lmn | 0.133±0.002ghi | 6.401±0.152nopq | 0.107±0.006klmn | 0.095±0.003qrs | — |
| 14-B44 | 0.335±0.026cd | 0.336±0.029a | 19.319±0.807a | 0.115±0.012klmn | 0.331±0.009def | 0.015±0.001c |
| 14-B45 | 0.165±0.016ijk | 0.151±0.008gh | 6.996±0.159nop | 0.047±0.002nop | 0.192±0.004ijkl | 0.001±0.002c |
| 14-B46 | 0.442±0.053b | 0.321±0.021a | 16.632±1.777cde | 0.014±0.002qr | 0.174±0.025jkl | — |
| 14-B47 | 0.249±0.005fg | 0.225±0.002cde | 13.286±0.207gh | 0.097±0.002klmn | 0.304±0.006efg | — |
| 14-B50 | 0.309±0.016de | 0.272±0.008b | 13.905±0.056fg | 0.458±0.007e | 0.296±0.007efg | 0.113±0.001a |
| 14-B51 | 0.080±0.001opq | 0.096±0.003ijk | 3.683±0.059st | 0.042±0.001opq | 0.292±0.005efg | — |
| 14-B53 | 0.130±0.018lmn | 0.128±0.020hij | 4.643±0.708qrs | 0.025±0.003pqr | 0.117±0.018opq | 0.001±0.001c |
| 14-B54 | 0.364±0.048c | 0.319±0.018a | 15.032±0.260def | 0.215±0.011hi | 0.474±0.009bc | — |
| 14-B59 | 0.082±0.017pqr | 0.070±0.011mno | 2.898±0.588tu | 0.072±0.013opq | 0.125±0.029s | — |
| 14-B60 | 0.191±0.010hij | 0.199±0.002ef | 10.756±0.292i | 0.174±0.004hij | 0.091±0.004rs | 0.003±0.000c |
| 14-B61 | 0.099±0.002mno | 0.110±0.001ijk | 6.406±0.074nop | 0.240±0.002gh | 0.235±0.204hij | 0.097±0.163ab |
| 注:不同字母表示差异显著(P < 0.05)。下同。 Note:Different letters indicate significant difference at 0.05 level. The same as follows. |
||||||
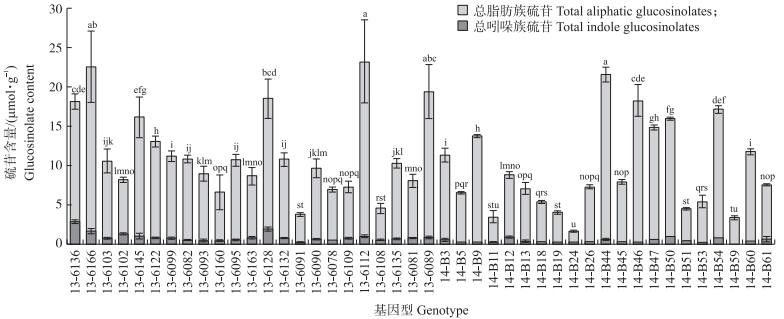
|
图 2 萝卜肉质根中总脂肪族硫苷、总吲哚族硫苷和总硫苷的含量 Fig. 2 Total content of aliphatic, indole and total glucosinolates(GLS)in radish taproot |
进一步对单一硫苷组分含量分析, 发现检测到的8种脂肪族硫苷(GRE、PRO、RAA、n-Heptyl、GIV、GBN、GRH、GER)含量差异显著, 其中GRH含量最高, 在基因型13-6112中高达21.25 μmol · g-1, 在基因型14-B5中最低为1.27 μmol · g-1。此外, 不同萝卜种质中的硫苷均以脂肪族硫苷为主, 占总硫苷含量的84%~99%。4种吲哚族硫苷(4OH、GBC、4ME、NEO)含量则相对较低, 平均含量仅为0.62 μmol · g-1, 含量最高的种质是基因型13-6136(2.78 μmol · g-1), 含量最低的种质为基因型14-B59(0.11 μmol · g-1)。吲哚族硫苷总含量占总硫苷含量比例为1.03%~15.32%。因此, 不同种质萝卜肉质根中单一硫苷组分之间差异明显, 表明萝卜肉质根中硫苷含量差异与种质关系紧密。
通过结果分析, 共筛选到4份4-甲基亚磺酰丁基硫苷(RAA)含量较高的种质:13-6102、13-6135、13-6122、13-6081, 肉质根均为椭圆形, 其中13-6102和13-6122为红心萝卜, 13-6135和13-6081为红皮萝卜。4-甲硫基-3-丁烯基硫苷(GRH)含量影响肉质根辛辣味, 筛选到GRH含量较低种质4份(13-B24、13-6091、13-B59、13-B11), 为青皮萝卜。
2.3 萝卜肉质根硫苷含量的主成分分析通过对单一硫苷含量相关系数的矩阵分析(表 3)可知, 总硫苷含量与GBN、GER、GRH含量呈极显著正相关, 与RAA、GBC、4ME、NEO含量呈显著正相关; 单一硫苷之间存在不同程度的相关性, 同族硫苷间相关性较强, 如GRE与PRO之间呈极显著正相关(相关系数0.998)。不同族硫苷之间也存在相关性, 如GBC与GER、GRH相关系数分别为0.502和0.509, 均呈显著正相关。
| 硫苷 Glucosinolate |
GRE | PRO | n-Heptyl | RAA | GIV | 4OH | GBN | GER | GRH | GBC | 4ME | NEO | T-GLS |
| GRE | 1.000 | ||||||||||||
| PRO | 0.998** | 1.000 | |||||||||||
| n-Heptyl | 0.629** | 0.609** | 1.000 | ||||||||||
| RAA | 0.157 | 0.158 | 0.433* | 1.000 | |||||||||
| GIV | -0.237 | -0.205 | -0.085 | 0.194 | 1.000 | ||||||||
| 4OH | 0.284 | 0.293 | 0.361 | 0.462* | 0.065 | 1.000 | |||||||
| GBN | -0.148 | -0.142 | -0.058 | 0.282 | 0.384 | 0.040 | 1.000 | ||||||
| GER | -0.048 | -0.055 | 0.289 | 0.572* | 0.289 | 0.200 | 0.778** | 1.000 | |||||
| GRH | -0.145 | -0.149 | 0.165 | 0.477* | 0.388 | 0.045 | 0.844** | 0.954** | 1.000 | ||||
| GBC | -0.203 | -0.223 | 0.153 | 0.333 | -0.090 | -0.244 | 0.211 | 0.502* | 0.509* | 1.000 | |||
| 4ME | -0.085 | -0.096 | 0.158 | 0.549* | -0.074 | -0.179 | 0.400 | 0.602** | 0.568* | 0.764** | 1.000 | ||
| NEO | -0.027 | -0.029 | 0.299 | 0.019 | 0.390 | 0.060 | 0.261 | 0.432* | 0.488* | 0.397 | 0.203 | 1.000 | |
| T-GLS | -0.083 | -0.088 | 0.243 | 0.522* | 0.355 | 0.053 | 0.813** | 0.961** | 0.993** | 0.574* | 0.625* | 0.510* | 1.000 |
| Note:*P < 0.05, * *P < 0.01. | |||||||||||||
通过主成分分析得到4个特征值大于1的主成分, 4个特征根的方差贡献率分别为39.99%、22.29%、12.25%和8.66%。由上述4个变量可代替原有的12个变量, 通过特征根得到的特征向量乘以标准化变量得出主成分表达式。第1主成分代表GBN、GER、GRH、GBC、4ME和NEO这6种硫苷的信息, 第2主成分代表GRE、PRO和RAA这3种硫苷的信息, 2个主成分可代表大部分硫苷信息。由前2个特征根所对应的特征向量得出由标准化变量表达的单个主成分的关系式分别如下:
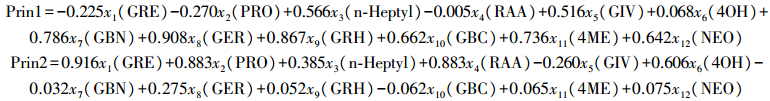
|
在主成分1和2表达式中, GRE、PRO、n-Heptyl、GER、GRH的特征向量较大, 表明萝卜肉质根以脂肪族硫苷为主。根据不同萝卜品种的主成分得分值较高的为基因型13-6112、13-6166、14-B44, 最低的为基因型14-B24, 这与表 2结果相一致。
3 讨论肉质根是萝卜重要的产品器官, 硫苷及其衍生物显著影响肉质根营养与风味品质, 准确鉴定种质硫苷含量, 通过杂交育种或基因工程技术定向改变硫苷含量组分, 以改良萝卜品质、提高其保健功效成为当前重要研究方向。本研究利用HPLC与LC-MS对萝卜肉质根中的硫苷组分和含量进行检测分析, 共鉴定到12种硫苷组分, 其中8种脂肪族硫苷, 4种吲哚族硫苷; 种质间硫苷组分也存在差异, 如在13-6091、13-6109等种质肉质根中未检测到NEO。另外, 本研究首次在萝卜肉质根中检测到n-Heptyl和GIV。在44份萝卜种质中均未检测到芳香族硫苷, 这与Ciska等[27]和Ishida等[25]研究结果一致。但在本研究中未检测到2-丙烯基硫苷和5-甲基亚磺酰戊基硫苷[22]以及3-丁烯基硫苷和2-苯基乙基硫苷[28], 推测可能与萝卜基因型及不同生长发育阶段有关。
硫苷在不同物种中具有多样性[2]。研究发现十字花科蔬菜中甘蓝类的硫苷含量最高, 是芥菜类和白菜类的10倍, 萝卜类的15倍[29]; 十字花科作物的硫苷组分和含量具有较大差异, 其主要影响因素是基因型差异[30]。白菜类作物苗期植株硫苷含量为1.77~42.71 μg · g-1, 存在一定的自然变异; 材料间硫苷含量差异最大达到1 127倍(3-丁烯基硫苷含量), 其他各硫苷组分含量材料间的变异幅度为18~152倍, 总硫苷含量最大相差51倍[31]。类似地, 在萝卜硫苷含量研究中也发现, 不同种质硫苷组分及含量不同。本研究通过对不同种质萝卜肉质根中硫苷的定量分析发现, GRH含量最高, 与前人的结果一致[22, 28]。44个萝卜种质肉质根总硫苷含量范围1.61~23.23 μmol · g-1, 高于Ciska等[27]的检测结果(1.9~7.9 mg · 100 g-1), 低于Ishida等[25]的检测结果(59.69~163.91 mg · 100 g-1, 7.9~117.6 μmol · g-1), 这可能是由于试验材料基因型和生长环境不同造成的。此外, 硫苷在一些十字花科蔬菜中的含量约占干质量的1%[1], 与本研究不同萝卜种质肉质根中硫苷总量占干质量比例(0.14%~0.97%)一致。
本试验主成分分析显示, 萝卜肉质根总硫苷含量与GBN、GER、GRH含量极显著正相关, 各硫苷含量之间存在一定程度的相关性。由于GRE、PRO、n-Heptyl、GER、GRH的特征向量较大, 暗示萝卜肉质根硫苷以脂肪族硫苷为主。RAA降解产物萝卜硫素诱导阶段Ⅱ解毒酶产生, 同时具有抑制乳腺癌的化学诱导作用。不同种质萝卜肉质根中硫苷含量差异显著, 硫苷含量高的品种可用作抗菌抗虫品种筛选; 筛选出4份RAA含量较高的萝卜种质均为红心品种, 表明红心萝卜中有益成分的硫苷组分(RAA)含量较高。4份GRH含量较低的萝卜种质以绿皮萝卜为主, 挥发性的GRH与萝卜肉质根辛辣味密切相关[32], 绿皮萝卜含影响辛辣味的硫苷(GRH)较少, 这与绿皮萝卜多为水果萝卜类型是一致的, 可用于筛选低辣味水果型品种。通过主成分分析得到各硫苷之间的相关性, 结合基因的表达及调控机制, 可找出单个硫苷含量的基因表达调控机制, 从而调节控制特定硫苷的含量。本研究结果为萝卜品质性状遗传改良与优良品种培育提供重要的种质资源和理论依据。
| [1] |
Blažević1 I, Montaut S, Bur Ač1 ul F, et al. Glucosinolates: novel sources and biological potential[M]//Mérillon J M, Ramawatk K G. Glucosinolates. Cham: Springer, 2015: 1-58.
|
| [2] |
Mithen R F, Dekker M, Verkerk R, et al. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods[J]. Journal of the Science of Food and Agriculture, 2000, 80(7): 967-984. DOI:10.1002/(ISSN)1097-0010 |
| [3] |
Clay N K, Adio A M, Denoux C, et al. Glucosinolate metabolites required for an Arabidopsis innate immune response[J]. Science, 2009, 323: 95-101. DOI:10.1126/science.1164627 |
| [4] |
王会敏, 徐克. 异硫氰酸酯抗肿瘤作用机制研究新进展[J]. 中国肺癌杂志, 2017, 20(3): 213-218. Wang H M, Xu K. Advances in research of antitumor mechanisms of isothiocyanates[J]. Chinese Journal of Lung Cancer, 2017, 20(3): 213-218 (in Chinese with English abstract). |
| [5] |
Bassan P, Bhushan S, Kaur T, et al. Extraction, profiling and bioactivity analysis of volatile glucosinolates present in oil extract of Brassica juncea var. raya[J]. Physiology and Molecular Biology of Plants, 2018, 24(3): 399-409. DOI:10.1007/s12298-018-0509-4 |
| [6] |
Agerbirk N, Olsen C E. Glucosinolate structures in evolution[J]. Phytochemistry, 2012, 77: 16-45. DOI:10.1016/j.phytochem.2012.02.005 |
| [7] |
Halkier B A, Gershenzon J. Biology and biochemistry of glucosinolates[J]. Annual Review of Plant Biology, 2006, 57: 303-333. DOI:10.1146/annurev.arplant.57.032905.105228 |
| [8] |
Redovnikovic I R, Glivetic T, Delonga K, et al. Glucosinolates and their potential role in plant[J]. Periodicum Biologorum, 2008, 110: 297-309. |
| [9] |
Kelly P J, Bones A, Rossiter J T, et al. Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea[J]. Planta, 1998, 206(3): 370-377. DOI:10.1007/s004250050412 |
| [10] |
Andreasson E, Jørgensen L B, Hoglund A, et al. Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus[J]. Plant Physiology, 2001, 127(4): 1750-1763. DOI:10.1104/pp.010334 |
| [11] |
Husebye H, Chadchawan S, Winge P, et al. Guard cell-and phloem idioblast-specific expression of thioglucoside glucohydrolase 1(myrosinase)in Arabidopsis[J]. Plant Physiology, 2002, 128: 1180-1188. DOI:10.1104/pp.010925 |
| [12] |
Reichelt M, Brown P D, Schneider B, et al. Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana[J]. Phytochemistry, 2002, 59: 663-671. DOI:10.1016/S0031-9422(02)00014-6 |
| [13] |
Mellon F A, Bennett R N, Holst B, et al. Intact glucosinolate analysis in plant extracts by programmed cone voltage electrospray LC/MS:performance and comparison with LC/MS/MS methods[J]. Analytical Biochemistry, 2002, 306(1): 83-91. |
| [14] |
Millán S, Sampedro M C, Gallejones P, et al. Identification and quantification of glucosinolates in rapeseed using liquid chromatography-ion trap mass spectrometry[J]. Analytical and Bioanalytical Chemistry, 2009, 394(6): 1661-1669. DOI:10.1007/s00216-009-2823-8 |
| [15] |
Ediagea E N, Mavungua J D D, Scippo M L, et al. Screening, identification and quantification of glucosinolates in black radish(Raphanus sativus L. niger)based dietary supplements using liquid chromatography coupled with a photodiode array and liquid chromatography-mass spectrometry[J]. Journal of Chromatography A, 2011, 1218: 4395-4405. DOI:10.1016/j.chroma.2011.05.012 |
| [16] |
Kim H J, Lee M J, Jeong M H, et al. Identification and quantification of glucosinolates in kimchi by liquid chromatography-electrospray tandem mass spectrometry[J]. International Journal of Analytical Chemistry, 2017, 2017: 1-8. |
| [17] |
Oh S K, Tsukamoto C, Kim K W, et al. Investigation of glucosinolates, and the antioxidant activity of Dolsan leaf mustard kimchi extract using HPLC and LC-PDA-MS/MS[J]. Journal of Food Biochemistry, 2017, 41(3): e12366. DOI:10.1111/jfbc.2017.41.issue-3 |
| [18] |
Augustine R, Bisht N C. Biotic elicitors and mechanical damage modulate glucosinolate accumulation by co-ordinated interplay of glucosinolate biosynthesis regulators in polyploid Brassica juncea[J]. Phytochemistry, 2015, 117: 43-50. DOI:10.1016/j.phytochem.2015.05.015 |
| [19] |
代梅, 高林, 吴继红. 西兰花中硫代葡萄糖苷的研究进展[J]. 食品研究与开发, 2017, 38(6): 212-217. Dai M, Gao L, Wu J H. Research progress on the glucosinolates in broccoli[J]. Food Research and Development, 2017, 38(6): 212-217 (in Chinese with English abstract). DOI:10.3969/j.issn.1005-6521.2017.06.048 |
| [20] |
Xu R, Kong W W, Peng Y F, et al. Identification and expression pattern analysis of the glucosinolate biosynthetic gene BoCYP83b1 from broccoli[J]. Biologia Plantarum, 2018, 62(3): 521-533. DOI:10.1007/s10535-018-0797-0 |
| [21] |
李秋云, 戴绍军, 陈思学, 等. 萝卜芥子油苷组分及含量的分析[J]. 园艺学报, 2008, 35(8): 1205-1208. Li Q Y, Dai S J, Chen S X, et al. Analysis of glucosinolate composition and content in radish[J]. Acta Horticulturae Sinica, 2008, 35(8): 1205-1208 (in Chinese with English abstract). DOI:10.3321/j.issn:0513-353X.2008.08.017 |
| [22] |
杨丽娟, 周胜军, 毛伟海, 等. 萝卜叶片和肉质根硫代葡萄糖苷组分与含量分析[J]. 浙江农业学报, 2010, 22(3): 311-316. Yang L J, Zhou S J, Mao W H, et al. Composition and content of glucosinolates in leaves and edible roots of radish[J]. Acta Agriculturae Zhejiangensis, 2010, 22(3): 311-316 (in Chinese with English abstract). DOI:10.3969/j.issn.1004-1524.2010.03.009 |
| [23] |
Ishida M, Nagata M, Ohara T, et al. Small variation of glucosinolate composition in Japanese cultivars of radish(Raphanus sativus L.)requires simple quantitative analysis for breeding of glucosinolate component[J]. Breeding Science, 2012, 62: 63-70. DOI:10.1270/jsbbs.62.63 |
| [24] |
袁伟玲, 袁尚勇, 崔磊, 等. 水果萝卜肉质根和叶片硫代葡萄糖苷鉴定及含量分析[J]. 中国蔬菜, 2017(11): 27-32. Yuan W L, Yuan S Y, Cui L, et al. Identification and content analysis of glucosinolates in leaf and root of fruity radish(Raphanus stivus L.)[J]. China Vegetables, 2017(11): 27-32 (in Chinese with English abstract). |
| [25] |
Ishida M, Kakizaki T, Ohara T, et al. Development of a simple and rapid extraction method of glucosinolates from radish roots[J]. Breeding Science, 2011, 61: 208-211. DOI:10.1270/jsbbs.61.208 |
| [26] |
Matthäus B, Luftmann H. Glucosinolates in members of the family Brassicaceae:separation and identification by LC/ESI-MS-MS[J]. Journal of Agricultural and Food Chemistry, 2000, 48: 2234-2239. DOI:10.1021/jf991306w |
| [27] |
Ciska E, Martyniak-Przybyszewska B, Kozlowska H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions[J]. Journal of Agricultural and Food Chemistry, 2000, 48: 2862-2867. DOI:10.1021/jf981373a |
| [28] |
张丽, 何洪巨, 陈静华, 等. 不同萝卜品种中硫代葡萄糖苷组分及含量分析[J]. 中国蔬菜, 2010(18): 43-46. Zhang L, He H J, Chen J H, et al. Glucosinolate composition and content analysis of different radish(Raphanus sativus L.)varieties[J]. China Vegetables, 2010(18): 43-46 (in Chinese). |
| [29] |
孙秀波, 慕美财, 李玫瑰, 等. 十字花科蔬菜硫代葡萄糖苷含量比较[J]. 安徽农学通报, 2007, 13(19): 64-65. Sun X B, Mu M C, Li M G, et al. Comparison of glucosinolates in cruciferous vegetables[J]. Anhui Agricultural Science Bulletin, 2007, 13(19): 64-65 (in Chinese). DOI:10.3969/j.issn.1007-7731.2007.19.032 |
| [30] |
Schonhof I, Krumbein A, Brückner B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower[J]. Nahrung, 2004, 48(1): 25-33. DOI:10.1002/(ISSN)1521-3803 |
| [31] |
徐东辉.白菜类作物硫代葡萄糖甙及一些主要代谢组分的遗传分析[D].北京: 中国农业科学院, 2007. Xu D H. Genetic dissecton of glucosinolates and some metabolites in Brassica rapa[D]. Beijing: Chinese Academy of Agricultural Sciences, 2007(in Chinese with English abstract). |
| [32] |
李鲜, 陈昆松, 张明方, 等. 十字花科植物中硫代葡萄糖苷的研究进展[J]. 园艺学报, 2006, 33(3): 675-679. Li X, Chen K S, Zhang M F, et al. Research advance of glucosinolates from crucifer[J]. Acta Horticulturae Sinica, 2006, 33(3): 675-679 (in Chinese with English abstract). DOI:10.3321/j.issn:0513-353X.2006.03.054 |




