文章信息
- 汤小美, 林天元, 周珊珊, 李国凤, 刘普, 叶振风, 吴中营, 王东升, 朱立武
- TANG Xiaomei, LIN Tianyuan, ZHOU Shanshan, LI Guofeng, LIU Pu, YE Zhenfeng, WU Zhongying, WANG Dongsheng, ZHU Liwu
- 梨树根腐病病原菌的分离与鉴定及有效防控药剂筛选
- Pathogen identification of root rot in pear plant and fungicide screening for its efficient control
- 南京农业大学学报, 2017, 40(1): 76-83
- Journal of Nanjing Agricultural University (Social Science), 2017, 40(1): 76-83.
- http://dx.doi.org/10.7685/jnau.201607002
-
文章历史
- 收稿日期: 2016-07-01
2. 河南省农业科学院园艺研究所, 河南 郑州 450002
2. Institute of Horticulture, Henan Academy of Agricultural Sciences, Zhengzhou 450002, China
梨树根腐病是梨树种植过程中一种危害比较严重的根部病害,可造成根系腐烂,引起树势衰弱,甚至整株死亡[1]。近两年来,在我国黄河故道地区,出现部分梨树叶片黄化、叶缘焦枯、整株落叶、植株枯死现象,最初被当作缺素症矫治未见效果,致使有的梨园发病植株死亡率高达20%以上,给生产造成严重的经济损失。因此,明确该致病菌的种类,筛选出高效的防控药剂,方能对该病害发生进行有效防控。与传统的形态学鉴定相比,分子生物学技术能弥补形态学鉴定的不足,比较科学地反映病原菌的系统发育情况[2]。目前普遍应用的是rDNA-ITS分子序列分析和形态学鉴定相结合的方法,例如大豆[3-4]、山核桃[5]、水稻[6]根腐病及牡丹黑斑病[7]的病原菌鉴定。在梨树病害研究方面,炭疽病菌(Colletotrichum spp.)[8]、腐烂病菌(Valsa pyri)[9]、黑斑病菌(Alternaria alternate)[10]等也通过rDNA-ITS序列和同源序列分析比对的方法鉴定出致病菌种类。引起梨树根系腐烂的病原菌较多,然而,迄今还未见腐皮镰刀菌(Nectria haematococca,无性态Fusarium solani)引起梨树烂根死亡的报道。笔者对发病梨园分离的菌株进行形态学观察及rDNA-ITS序列分析鉴定,对其致病性进行试验,进行菌丝生长、孢子萌发有效抑制化学药剂的筛选,并对部分药剂进行田间防效试验,为该病害的防治提供试验依据。
1 材料与方法 1.1 试验材料2014年8月27日,从国家梨产业技术体系郑州综合试验站核心示范园取‘中梨一号’(又名‘绿宝石’)发病根系样品,带回实验室进行分离。抑菌药剂筛选试验共选用18种生产上常用杀菌剂(表 1)。
| 药剂 Fungicides | 有效成分 Effective components | 生产厂家 Manufacturer | 稀释倍数 Dilution times | 菌丝生长抑制率/% Inhibition rate of mycelium growth | 孢子萌发抑制率/% Inhibition rate of spores germination |
| 清水对照 Contrast | — | — | 0 | 0 | 0 |
| 25%三唑酮可湿性粉剂 25% triadimefon SP | 三唑酮 Triadimefon | 山东罗邦生物农药有限公司 Shandong Robond Biological Pesticide Co.,Ltd. | 500 | 43.72e | 43.74c |
| 72%霜脲·锰锌可湿性粉剂 72% cymoxanil+mancozeb SP | 霜脲氰、代森锰锌 Cymoxanil,mancozeb | 京博农化科技股份有限公司 Jingbo Agrochem Polytron Technologies Inc | 600 | 84.69a | 100.00a |
| 1.8%辛菌胺醋酸盐水剂 1.8% ametoctradin acetate SC | 辛菌胺醋酸盐 Ametoctradin acetate | 潍坊奥丰作物病害防治有限公司 Weifang Everrich Crop Disease Control Co. Ltd. | 400 | 85.58a | 100.00a |
| 80%波尔多液可湿性粉剂 80% bordeaux mixture SP | 碱式硫酸铜 Copper sulfate | 美国仙农有限公司 United States Shannon Co.,Ltd. | 400 | 15.45h | 100.00a |
| 50%福美双可湿性粉剂 50% thiram SP | 多菌灵 Carbendazim | 山东罗邦生物农药有限公司 Shandong Robond Biological Pesticide Co.,Ltd. | 600 | 65.74bc | 100.00a |
| 70%甲基硫菌灵可湿性粉剂 70% bordeaux mixture SP | 甲基硫菌灵 Bordeaux mixture | 江苏龙灯化学有限公司 Jiangsu Dragon Chemical Co.,Ltd. | 800 | 56.56cd | 85.60ab |
| 2.6%靓果安水剂 2.6% alkaloid fungicides SC | 生物碱 Alkaloid | 潍坊奥丰作物病害防治有限公司 Weifang Everrich Crop Disease Control Co. Ltd. | 800 | 2.37i | 28.61d |
| 30%矿物油·石硫微乳剂 30% mineral oil calcium polysulfide ME | 矿物油石硫 Mineral oil calcium polysulfide | 成都市新津生化工程研究所 Xinjin Chengdu Biochemical Engineering Research Institute | 400 | 26.61g | 82.99b |
| 2.1%青枯立克水剂 2.1% chlorogenic acid SC | 绿原酸 Chlorogenic acid | 西安嘉科农化有限公司 Xi′an Jiake Agrochemical Co. Ltd. | 500 | 6.16hi | 60.00c |
| 45%石硫合剂晶体 45% calcium polysulfide CS | 多硫化钙 Calcium polysulfide | 湖北省宜昌山峡农药厂 Hubei Province Yichang Pesticide Factory | 150 | 33.87fg | 98.79a |
| 99%噁霉灵水剂 99% hymexazol SC | 噁霉灵 Hymexazol | 吉林省延边西爱斯开化农药厂 Jilin Province Yanbian West Ace Kaihua Pesticide Factory | 4 000 | 39.25f | 20.24de |
| 60%苯醚甲环唑微乳剂 60% difenoconazole ME | 苯醚甲环唑 Difenoconazole | 海利尔药业集团股份有限公司 Hailier Pharmaceutical Group Limited by Share Ltd. | 1 000 | 68.81b | 82.34b |
| 95%吡唑醚菌酯乳油 95% pyraclostrobin EC | 吡唑醚菌酯 Pyraclostrobin | 广东德利生物科技有限公司 Guangdong Biological Technology Co.,Ltd. | 1 000 | 83.75a | 65.24c |
| 3%噻霉酮微乳剂 3% benziothiazolinone ME | 噻霉酮 Benziothiazolinone | 陕西西大华特科技实业有限公司 Shaanxi Xidahuate Technology Co.,Ltd. | 100 | 68.99b | 100.00 a |
| 21%过氧乙酸水剂 21% peracetic acid SC | 过氧乙酸 Peracetic acid | 石家庄宝峰化工有限公司 Shijiazhuang Baofeng Chemical Co. Ltd. | 800 | 1.11i | 12.33ef |
| 80%代森锰锌可湿性粉剂 80% mancozeb SP | 代森锰锌 Mancozeb | 印度印的菲尔工业有限公司 India′s Phil Industrial Co.,Ltd. | 800 | 85.31a | 100.00a |
| 75%百菌清可湿性粉剂 75% chlorothalonil SP | 百菌清 Chlorothalonil | 先正达集团有限公司 Syngenta Group Co.,Ltd. | 500 | 61.90bcd | 100.00a |
| 2%溃腐灵水剂 2% rot root fungicides SC | 苍术素、厚朴酚 Rhizoma atractylodis, honokiol | 潍坊奥丰作物病毒防治有限公司 Weifang Everrich Crop Disease Control Co. Ltd. | 100 | 5.30i | 58.08c |
| 注:数值后上标小写字母表示差异达到显著水平(P<0.05)。Different lowercase indicates significant difference at 0.05 level. The same as follows. | |||||
在灭菌的超净工作台上将发病根系样品用无菌水清洗,并用75%(体积分数)的乙醇消毒,之后用解剖刀切取病健交界处组织,置于PDA培养基平板上,25 ℃恒温培养。待菌丝产孢后,将纯化的分生孢子液用灭菌水稀释成在低倍显微镜下(目镜20×物镜10)孢子密度为每视野20~40个,在高倍显微镜下用接种环蘸取适量的孢子液,在PDA培养基上划线培养,12 h后选取单个分生孢子萌发形成的菌丝,移入另一PDA培养基上培养,获得病原菌的单孢纯化菌株[11]。
1.3 病原菌的形态特征观察每天观察并记录菌落的生长情况;用Olympus BX51显微镜观察菌丝与分生孢子的形态,并测量菌丝直径及分生孢子的形状与大小。用2%葡萄糖溶液制备孢子悬浮液,悬浮液浓度为1.0×106 CFU · mL-1,观察分生孢子萌发情况。
1.4 病原菌的分子生物学鉴定用真菌基因组DNA提取试剂盒(北京艾德莱生物科技有限公司)提取病原菌株的DNA,用通用引物 ITS1/ITS4对病菌核糖体DNA内部转录间隔区ITS序列进行PCR扩增,扩增引物序列分别为ITS1:5′-TCCG- TAGGTGAACCTGCGG-3′;ITS4:5′-TCCTCCGCTTATTGATATGC-3′[12-13]。PCR扩增体系为:rTaq酶10 μL,无菌水7 μL,上、下游引物各1 μL,模板DNA1 μL。PCR反应程序为:94 ℃ 3 min;94 ℃1 min,55 ℃ 30 s,72 ℃1 min,30个循环;72 ℃10 min[14]。凝胶回收的PCR产物连接到PGEM-Teasy载体上,并转化到大肠杆菌菌株DH5α感受态细胞中,将转化产物涂布于含Amp+(氨苄青霉素)的LB平板上,于37 ℃过夜培养,挑选单菌落PCR扩增鉴定阳性克隆,菌液送至华大基因科技有限公司测序。
1.5 病原菌的致病性试验将分生孢子制成浓度为1.0×106 CFU · mL-1的孢子悬浮液100 mL,并将PDA固体培养基上的菌丝块(2 g)捣碎制成100 mL溶液,选取长势健壮的‘翠冠’梨盆栽植株,以孢子和菌丝溶液等量混合液200 mL进行灌根接种,以无菌水为对照,3次重复,观察发病情况。待处理植株发病后,取根部发病组织再次进行病原菌分离纯化与测序鉴定。
1.6 rDNA-ITS序列与系统发育分析在GenBank网站(http://www.ncbi.nlm.nih.gov)的数据库中进行测序结果的比对,在线搜索同源性较高的已知序列,用软件Clustal X 1.83对序列进行比对,去掉两端的引物序列,利用BioEdit程序与已知病原菌的ITS区序列进行同源比对分析,并利用MEGA 3.1 软件以非加权配对算术平均法(unweighted pair-group method using arithmetic average,UPGMA)构建系统聚类树,分析其亲缘关系[15]。根据比对结果,并结合病原菌的形态特征、培养性状及致病性,对病原菌进行分析鉴定[16]。
1.7 常用杀菌剂抑菌试验在无菌条件下,采用改良平板对峙法[8],每个处理设6个重复,以无菌水为对照。每隔24 h观察记录1次并用十字交叉法测量菌落直径,第6天统计各处理菌丝的生长抑制率。菌丝生长抑制率=(CD-TD)/CD×100%。其中:CD为对照组菌落净生长量;TD为药剂处理组菌落净生长量。
吸取浓度为1.0×106 CFU · mL-1的孢子悬浮液40 μL,处理组加供试杀菌剂推荐使用浓度的两倍液40 μL,对照用无菌水代替杀菌剂,充分混匀后滴于洁净的凹玻片上,于无菌培养皿中保湿,25 ℃恒温培养,培养4 h后,在Olympus BX51显微镜下观察,统计各处理分生孢子萌发情况,参照文献[17]计算孢子萌发抑制率。
1.8 部分杀菌剂的室内毒力试验与田间试验选择抑菌效果好的4种药剂做进一步的毒力测定试验,按照2倍法进行梯度稀释[18],80%代森锰锌浓度梯度:500、250、125和62.5 μg · mL-1,1.8%辛菌胺醋酸盐:180、90、45和22.5 μg · mL-1,95%吡唑醚菌酯乳油:125、62.5、31.25和15.625 μg · mL-1,72%霜脲 · 锰锌:600、300、150和75 μg · mL-1,培养6 d后测量菌落直径,并计算各处理菌丝生长抑制率,建立毒力回归方程[19],依据最小二乘法求出EC50值。萌芽前以药剂推荐浓度对田间发病植株进行灌根处理,根据树冠投影面积确定用药量(5 kg · m-2),以无菌水为对照,抽查枯焦叶片计算田间实际防效[20]。
2 结果与分析 2.1 植株发病症状与病原菌分离纯化及其形态学特征2014年8月27日,在郑州综合试验站核心示范园考察发现,部分‘中梨一号’梨树植株发病较重,发病初期植株成熟叶片叶缘枯焦,进而叶片枯死脱落,又发出新叶,严重发病植株则出现整株死亡,而未发病植株叶色正常(图 1)。我们从采集的发病植株根系样品中,分离获得4株纯化菌株,依次编号为FSZL1~FSZL4,经培养,观察,比较其菌丝生长情况、孢子形态、ITS序列分析,确定这4个菌株为同一致病菌。

|
图 1 梨树根腐病田间发病植株特征 Figure 1 Symptoms of Fusarium solani in diseased pear plant A. 正常植株 Normal plant;B. 发病初期,成熟叶缘枯焦 Margin scorch of mature leaf at diseased early stage;C. 发病中期,叶片枯死脱落,再次长出新叶 Dead leaves fall and new ones come out at diseased middle stage;D. 发病严重植株根系 The died roots of severely diseased plant. |
菌落初期呈白色,之后呈淡红色至紫色,培养15 d之后,肉眼可见菌落中间出现白色分生孢子团(图 2-A)气生菌丝生长良好,菌丝平均生长速率为9.3 mm · d-1,在40×10倍的显微镜下观察,菌丝无色,具有隔膜和分枝,分枝一般较细,直径为2~5 μm(图 2-B);病原菌培养5 d后已经开始产生分生孢子,分生孢子梗长短不一,单瓶梗(图 2-C),可产生分生孢子座(图 2-D)。在40×10倍显微镜下观察,大型分生孢子为纺锤形至镰刀形,壁稍厚,3~5个隔膜,多为3个隔膜,大小为(29~38)μm×(4~5)μm,小分生孢子为椭圆形或卵形,大多单胞,极少数有1个隔膜,无色透明,大小为(6~15)μm×(2~4)μm(图 2-E);分生孢子在2%的葡萄糖溶液中培养4 h即可萌发(图 2-F),伸出芽管数目以1~2个居多,少数能长出3个,芽管出现的位置也不固定,有的芽管可以形成分枝,进而形成侵染丝(图 2-G)。菌丝培养15 d后开始产生厚垣孢子,厚垣孢子椭圆形至矩圆形,淡黄色,顶生或间生,单生或2个成串生长,厚垣孢子大小为6~10 μm (图 2-H)。4个菌株的这些形态学特征一致,与已研究报道过的腐皮镰刀菌(Fusarium solani)相同。
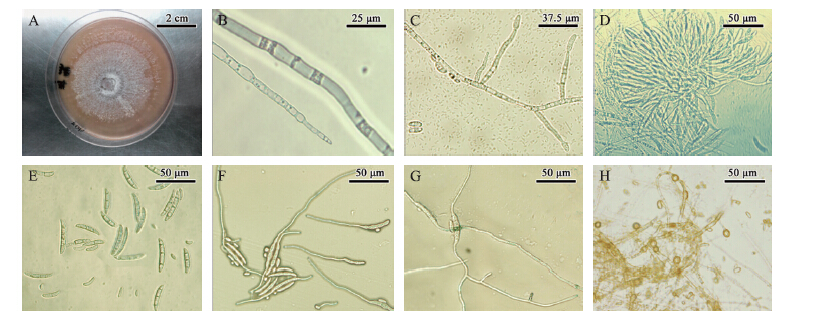
|
图 2 梨树根腐病致病菌菌丝、分生孢子及其萌发的形态特征 Figure 2 Morphological characteristics of mycelium,conidiophores and germination of pear root rot pathogen A.菌落外观形态Morphology of colony;B.菌丝显微结构Structure of mycelium;C.分生孢子梗及产孢细胞Conidiophore peduncle and conidiogenous cell;D.分生孢子座Sporodochium;E.孢子形态Morphology of conidial;F.分生孢子萌发形态Morphology of conidiophores germination;G.芽管形成分枝The formation of branches on germ tube;H.厚垣孢子形态Morphology of chlamydospores |
将分离纯化得到的菌株FSZL4孢子悬浮液接种到健康的盆栽‘翠冠’梨树根部(图 3),每个处理接种4盆,对照组每个处理2盆,每个处理重复3次,接种一段时间后观察,处理组有10盆‘翠冠’梨发病,且发病症状与田间发病症状相同,清水处理组均未发病。从接种发病的梨树根样上再次分离得到该病原菌,其培养性状与前期接种菌株的培养性状相同。依照柯赫氏法则(Koch′s postulate),接种的病菌分离物即为从‘中梨一号’根部中分离纯化的病原菌。
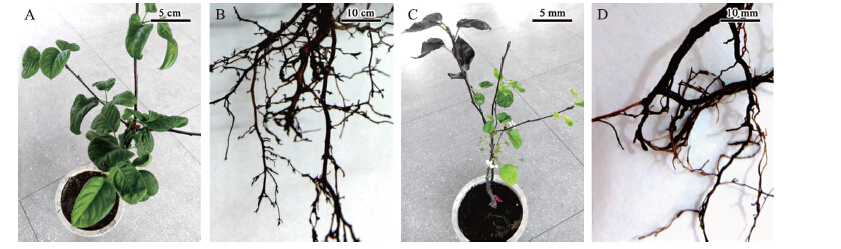
|
图 3 盆栽梨树根部接种FSZL4菌株分生孢子与菌丝后的发病症状 Figure 3 Symptoms of diseased root of potted pear plant inoculated with conidiophores and mycelia of FSZL4 A.对照植株叶片生长正常 Normal growth of control pear plant leaf;B.对照植株根系生长正常 Normal root system of control plant;C.接种发病植株落叶并二次开花 Defoliation and return bloom in diseased pear plant by pathogen inoculation;D.接种发病植株根系皮层腐烂 Rot root cortex of diseased pear plant |
分别以分离到的4个菌株基因组DNA做模板,用ITS1/ITS4进行PCR扩增,获得扩增片段长度约530 bp左右,测序分析结果4个菌株rDNA-ITS序列完全一致,GenBank登录号为:KU360148.1。
在NCBI网站中进行Web BLAST同源性比较,上述序列与日本桑树枝条上分离的腐皮镰刀菌(Nectria haematococca,无性态Fusarium solani)菌株MAFF 840047(AB513852.1)、北京紫花苜蓿根腐病菌株YQC-C5 (KF939490.1)、北京黄栌根腐病病原菌BJS2(AB369415.1)、美国加利福尼亚葡萄根腐病菌株AM(KR909201)、兰州百合根腐病菌株CBS 118521(KP091293.1)、甘肃宁夏枸杞根腐病菌株GSJT7(KM457086.1)、江苏橡树根腐病菌株xsd08086(FJ478128.1)的rDNA-ITS序列的一致性和比对覆盖率均为100%,而与意大利树莓根腐病菌株CBS 101018(KM231800.1,Neocosmospora rubicola)、日本百脉根根腐病菌株MAFF 240020(AB513846.1,Fusarium striatum)的同源性差别较大(图 4)。因此,可以进一步确定该病原菌为腐皮镰刀菌(Nectria haematococca,无性态Fusarium solani)。
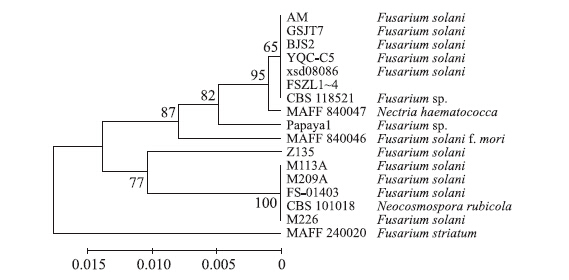
|
图 4 梨根腐病病原菌与同源性较高病原菌的rDNA-ITS序列系统聚类树 Figure 4 Phylogenetic tree of the pathogen of pear root rot based on rDNA-ITS cluster analysis FSZL1~FSZL4:河南梨树根系Henan,Chinese pear root;YQC-C5:北京紫花苜蓿Beijing,Medicago sativa;BJS2:北京黄栌Beijing,Cotinus coggygria Scop;AM:加利福尼亚葡萄California,Grapevine;CBS 118521:兰州百合Lanzhou,Lily;GSJT7:甘肃宁夏枸杞Gansu,Lycium barbarum; xsd08086:江苏橡树Jiangsu,Oak shoot;MAFF 840047:日本桑树Japan,Morus alba shoot;Papaya1:海南番木瓜Hainan,Carica papaya;FS-01403:中国人参Jilin,Chinese ginseng;Z135、M209A、M226、M113A:波兰马铃薯Poland,Solanum tuberosum L.;CBS 101018:意大利树莓Italy,Rubus idaeus;MAFF 240020:日本百脉根Japan,Lotus corniculatus var. japonicus root.步长值为0.005。Step value is 0.005. 分支左侧数值为聚类自举支持率。Bootstrap values are shown on the left of each clade. |
药剂筛选试验结果(表 1)表明,72%霜脲 · 锰锌可湿性粉剂600倍液、1.8%辛菌胺醋酸盐400倍液、95%吡唑醚菌酯乳油1 000倍液和80%代森锰锌可湿性粉剂800倍液,对菌丝生长的抑制率均在85%左右,且处理间差异未达到显著水平,而2.1%青枯立克水剂500倍液、80%波尔多液可湿性粉剂400倍液、2.6%靓果安水剂800倍液、30%矿物油 · 石硫微乳剂400倍液、21%过氧乙酸水剂800倍液和2%溃腐灵水剂100倍液抑菌率均较低。
不同药剂处理对梨树根腐病病原菌分生孢子萌发抑制效果差别很大。72%霜脲 · 锰锌可湿性粉剂600倍液、80%波尔多液可湿性粉剂400倍液、1.8%辛菌胺醋酸盐400倍液和80%代森锰锌可湿性粉剂800倍液对分生孢子萌发抑制率高达100%,2.1%青枯立克、3%噻霉酮微乳剂和2%溃腐灵有一定的抑菌作用,而99%噁霉灵、21%过氧乙酸和2.6%靓果安对分生孢子的萌发基本无抑制作用(表 1)。
2.5 部分杀菌剂的室内毒力测定试验与田间试验效果毒力试验结果表明,4种药剂毒力回归方程相关系数均达到极显著水平;腐皮镰刀菌对4种药剂的敏感性存在差异,95%吡唑醚菌酯和80%代森锰锌对该病原菌菌丝生长的毒力最强,72%霜脲 · 锰锌毒力居中,1.8%辛菌胺醋酸盐的毒力最差(表 2)。
| 抑菌药剂名称 Fungicides | 有效成分 Effective components | 毒力回归方程 Regression equation | 相关系数 Correlation coefficient | EC50/ (μg·mL-1) | 稀释倍数 Dilution times | 田间实际防效/% Effect on disease control in field |
| 80%代森锰锌可湿性粉剂 80% mancozeb SP | 代森锰锌 Mancozeb | Y=3.825+0.767x | 0.967 | 33.903c | 800 | 92.6a |
| 95%吡唑醚菌酯乳油 95% pyraclostrobin EC | 吡唑醚菌酯 Pyraclostrobin | Y=3.818+0.913x | 0.990 | 19.684c | 1 000 | 74.5b |
| 1.8%辛菌胺醋酸盐水剂 1.8% ametoctradin acetate SC | 辛菌胺醋酸盐 Ametoctradin acetate | Y=2.389+1.140x | 0.992 | 194.736a | 400 | 92.8a |
| 72%霜脲·锰锌可湿性粉剂 72% cymoxanil+mancozeb SP | 霜脲氰、代森锰锌 Cymoxanil,mancozeb | Y=3.508+0.818x | 0.995 | 66.612b | 600 | 92.3a |
田间试验结果表明,1.8%辛菌胺醋酸盐对根腐病病原菌的实际防效最好,达到92.8%,80%代森锰锌与72%霜脲 · 锰锌的实际防效分别为92.6%和92.3%(表 2),三者实际防效未达到显著差异。95%吡唑醚菌酯乳油的防效一般,仅为74.5%,与其他3种药剂防效差异达到显著水平(P<0.05)。发病梨树经药剂与无菌水(对照)灌根后的生长情况见图 5。

|
图 5 田间梨树发病植株萌芽前药剂灌根处理及对照 Figure 5 Diseased plants treated by irrigating fungicide before budding and the control in the field A:1.8%辛菌胺醋酸盐灌根处理植株 Irrigating solution of 1.8% ametoctradin acetate;B:对照植株 The control pear plant |
根据现有报道,造成梨树根系腐烂的病原菌多为白纹羽病菌(Rosellinia necatrix)和紫纹羽病菌(Helicobasidium mompa)[21]、梨树根朽病菌(Armillariella tabescens)[22]、疫霉菌(Phytophthora cactorum)[23]等,尤其是疫霉菌(Phytophthora cactorum)能侵染整个梨树植株,引起根腐、茎腐、基腐、果腐、树干溃疡、枝萎、叶腐、叶枯和早期落叶等症状,造成严重的经济损失。研究表明,腐皮镰刀菌(Fusarium solani)可以引起甘草等多种植物的根腐病[24],但是,该病原菌在梨树上造成的根腐病还是首次报道。
镰刀菌种类(Fusarium spp.)的分类方法,一般是根据形态学特征和分生孢子的培养特征,如菌丝的形态、色素及黏孢团的有无,分生孢子的形状、大小、分隔数、瓶梗类型、顶细胞的有无等。但有些学者指出,仅根据分生孢子的培养特征和形态特征分类系统可以分到不同的变种,他们认为镰刀菌的分类必须要用单孢分离的方法,并结合大孢子的形状、小孢子和厚垣孢子的有无等[25]。随着分子生物学技术的不断发展,镰刀菌的鉴定方法也在不断地变化,形态学特征与分子生物学相结合的方法是目前普遍认可的方法,应用分子生物学技术,能比较科学地反映出镰刀菌的系统发育情况,为镰刀菌菌种的快速鉴定提供了科学的理论依据[26-27]。本研究根据形态特征分析,并结合分子生物学鉴定的方法,确定引起黄河故道地区梨树根腐病的病原菌为腐皮镰刀菌(Nectria haematococca,无性态Fusarium solani),但该病原菌对梨树寄生是否具有专一性,尚待下一步试验研究。
3.2 室内药剂的筛选18种杀菌药剂筛选结果表明:80%代森锰锌、95%吡唑醚菌酯、1.8%辛菌胺醋酸盐和72%霜脲 · 锰锌抑制菌丝生长和孢子萌发效果均较好。通过室内毒力试验可以更清楚地了解杀菌剂对根腐病病原菌的有效抑制浓度,结果表明,95%吡唑醚菌酯和80%代森锰锌的EC50值较小,其次是72%霜脲 · 锰锌,1.8%辛菌胺醋酸盐的EC50值最大。虽然95%吡唑醚菌酯对菌丝生长的毒力比72%霜脲 · 锰锌和1.8%辛菌胺醋酸盐的强,但是田间试验结果表明,95%吡唑醚菌酯实际防效最差,这可能与95%吡唑醚菌酯对分生孢子萌发的抑制率较低有关。在实际生产用药中,我们还需要综合考虑药剂的成本、残留量等因素,相关问题还需要进一步的研究。
| [1] | Ma L J, Geiser D M, Proctor R H, et al. Fusarium pathogenomics[J]. Annual Review of Microbiology, 2013,67: 399–416. DOI: 10.1146/annurev-micro-092412-155650 |
| [2] | 肖荣风, 刘波, 林抗美, 等. 斑叶露兜树茎腐病病原鉴定及植物体内菌量测定[J]. 园艺学报, 2009, 36(2): 251–256. Xiao R F, Liu B, Lin K M, et al. Identification and distribution inside plant of pathogen causing stem rot of Pandanus veitchii[J]. Acta Horticulturae Sinica, 2009,36(2): 251–256. (in Chinese) |
| [3] | Costa S S, Matos K S, Tessmann D J, et al. Fusarium paranaense sp. nov., a member of the Fusarium solani species complex causes root rot on soybean in Brazil[J]. Fungal Biology, 2016,120(1): 51–60. DOI: 10.1016/j.funbio.2015.09.005 |
| [4] | Zhang J X, Xue A G, Zhang H J, et al. Response of soybean cultivars to root rot caused by Fusarium species[J]. Canadian Journal of Plant Science, 2010,90(5): 767–776. DOI: 10.4141/CJPS09133 |
| [5] | Zhang C Q, Liu Y H, Xu B C. First report of Fusarium root rot on Chinese hickory (Carya cathayensis) caused by Fusarium oxysporum in China[J]. Plant Disease, 2015,99(9): 1284–1286. |
| [6] | Balmas V, Corda P, Marcello A, et al. Fusarium nygamai associated with Fusarium foot rot of rice in Sardinia[J]. Plant Disease, 2000,84: 801–807. |
| [7] | 石良红, 赵兰勇, 吴迪, 等. 山东牡丹黑斑病的病原菌鉴定与ITS序列分析[J]. 园艺学报, 2015, 42(3): 585–590. Shi L H, Zhao L Y, Wu D, et al. The identification and analysis of ITS sequence on tree peony black spot in Shandong[J]. Acta Horticulturae Sinica, 2015,42(3): 585–590. (in Chinese) |
| [8] | 吴良庆, 朱立武, 衡伟, 等. 砀山梨炭疽病病原鉴定及其抑菌药剂筛选[J]. 中国农业科学, 2010, 43(18): 3750–3758. Wu L Q, Zhu L W, Heng W, et al. Identification of Dangshan pear anthracnose pathogen and screening fungicides against it[J]. Scientia Agricultura Sinica, 2010,43(18): 3750–3758. (in Chinese) |
| [9] | 周玉霞, 程栎菁, 张美鑫, 等. 我国梨腐烂病病原菌的初步鉴定及序列分析[J]. 果树学报, 2013, 30(1): 140–146. Zhou Y X, Cheng Y J, Zhang M X, et al. Sequence analysis and preliminary identification for the pathogen of pear Valsa canker in China[J]. Journal of Fruit Science, 2013,30(1): 140–146. (in Chinese) |
| [10] | 周求根, 李诚, 蒋军喜, 等. 贡梨果实黑斑病病原菌鉴定及室内防治药剂筛选[J]. 中国南方果树, 2013, 42(5): 35–38. Zhou Q G, Li C, Jiang J X, et al. Identification of the pathogen of pear black spot and indoor screening of fungicides[J]. South China Fruit, 2013,42(5): 35–38. (in Chinese) |
| [11] | 赵金梅, 高贵田, 古留杰, 等. 中华猕猴桃褐斑病病原鉴定及抑菌药剂筛选[J]. 中国农业科学, 2013, 46(23): 4916–4925. Zhao J M, Gao G T, Gu L J, et al. Identification and pharmaceutical screening of brown spot disease on Actinidia chinensis[J]. Scientia Agricultura Sinica, 2013,46(23): 4916–4925. (in Chinese) |
| [12] | 徐成楠, 王亚南, 胡同乐, 等. 蓝莓炭疽病病原菌鉴定及致病性测定[J]. 中国农业科学, 2014, 47(20): 3992–3998. Xu C N, Wang Y N, Hu T L, et al. Identification and pathogenicity of pathogen casuing anthracnose on Vaccinium[J]. Scientia Agricultura Sinica, 2014,47(20): 3992–3998. (in Chinese) |
| [13] | 黄欣阳, 刘志恒, 杨红, 等. 辣椒叶斑病的病原菌生物学特性研究[J]. 园艺学报, 2013, 40(2): 275–282. Huang X Y, Liu Z H, Yang H, et al. Biological characteristics of pepper leaf spot pathogen[J]. Acta Horticulturae Sinica, 2013,40(2): 275–282. (in Chinese) |
| [14] | 余磊, 赵建荣, RarisaraI, 等. 蓝莓枯枝病病原菌鉴定[J]. 植物病理学报, 2013, 43(4): 421–425. Yu L, Zhao J R, Rarisara I, et al. Identification of the pathogen causing twigs and stem dieback in blueberry[J]. Acta Phytopathologica Sinica, 2013,43(4): 421–425. (in Chinese) |
| [15] | 彭兴民, 吴疆翀, 郑益兴, 等. 云南引种印楝实生种群的表型变异[J]. 植物生态学报, 2012, 36(6): 560–571. Peng X M, Wu J C, Zheng Y X, et al. Phenotypic variation in cultivated populations of Azadirachta indica in Yunnan, China[J]. Chinese Journal of Plant Ecology, 2012,36(6): 560–571. (in Chinese) |
| [16] | 陈霞, 刘东, 张艳菊, 等. 黄瓜枯萎病病株镰孢菌的分离与鉴定[J]. 东北农业大学学报, 2010, 41(7): 37–44. Chen X, Liu D, Zhang Y J, et al. Isolation and identification of Fusarium from cucumber wilt plants[J]. Journal of Northeast Agricultural University, 2010,41(7): 37–44. (in Chinese) |
| [17] | 管磊, 郭贝贝, 王晓坤, 等. 苯醚甲环唑和氟啶胺的两种制剂包衣种子对花生土传真菌病害的防治效果[J]. 中国农业科学, 2015, 48(11): 2176–2186. Guan L, Guo B B, Wang X K, et al. Control efficacies of two preparations of difenoconazole and fluazinam by seed-coating against peanut soil-borne fungal diseases[J]. Scientia Agricultura Sinica, 2015,48(11): 2176–2186. (in Chinese) |
| [18] | 李荣峰, 徐秉良, 梁巧兰, 等. 白菜型冬油菜根腐病病原鉴定及室内毒力测定[J]. 甘肃农业大学学报, 2012, 47(5): 98–104. Li R F, Xu B L, Liang Q L, et al. Identification of root pathogen of winter turnip rapeseed and toxicity measurement[J]. Journal of Gansu Agricultural University, 2012,47(5): 98–104. (in Chinese) |
| [19] | 潘龙其, 张丽, 袁庆华, 等. 不同杀菌剂对拟枝孢镰刀菌的毒力测定及田间防效[J]. 中国农业大学学报, 2016, 21(1): 87–96. Pan L Q, Zhang L, Yuan Q H, et al. Toxicity measurement and field control of alfalfa root rot Fusarium sporotrichioide[J]. Journal of China Agricultural University, 2016,21(1): 87–96. (in Chinese) |
| [20] | 石爱丽, 邢占民, 郭玉炜, 等. 杀菌剂与杀虫剂配合施用的黄芪根腐病田间防效评价[J]. 河北农业科学, 2016, 20(1): 55–59. Shi A L, Xing Z M, Guo Y W, et al. Field control effect of combination of fungicide and insecticide on root rot disease of Astragalusm embranaceus[J]. Journal of Hebei Agricultural Sciences, 2016,20(1): 55–59. (in Chinese) |
| [21] | 涂勇. 果树主要根部病害及其防治方法研究进展[J]. 江苏农业科学, 2012, 40(10): 132–134. Tu Y. The research progress in control methods and main diseases of fruits[J]. Journal of Jiangsu Agricultural Sciences, 2012,40(10): 132–134. (in Chinese) |
| [22] | 杨智勇. 观赏果树根部病害的发生规律及综合防治[J]. 陕西农业科学, 2005(3): 85–88. Yang Z Y. The integrated control and occurred discipline of ornamental fruit trees root disease[J]. Journal of Shaanxi Agricultural Sciences, 2005(3): 85–88. (in Chinese) |
| [23] | Whiley A W. 油梨疫霉根腐病的杀菌防治及其对植株水分、产量和环颈病的影响[J]. 云南热作科技, 1988,11(4): 43–46. |
| [24] | 曹雪梅, 李生兵, 张惠玲, 等. 甘草根腐病病原菌鉴定[J]. 植物病理学报, 2014, 44(2): 213–216. Cao X M, Li S B, Zhang H L, et al. Identification of the pathogens causing root rot in Glycyrrhiza uralensis Fisch[J]. Acta Phytopathologica Sinica, 2014,44(2): 213–216. (in Chinese) |
| [25] | Snyder W C, Hansen H N. The species concept in Fusarium with reference to discolor and other section[J]. American Journal of Botany, 1945,32: 657–666. DOI: 10.2307/2437621 |
| [26] | Thangavelu R, Jayanthi A. RFLP analysis of rDNA-ITS regions of native non-pathogentic Fusarium oxysporum isolates and their field evalution for the suppression of Fusarium wilt disease of banana[J]. Australasian Plant Pathology, 2009,38: 13–21. DOI: 10.1071/AP08071 |
| [27] | Li S, Hartman G L. Molecular detection of Fusarium solani f. sp. glycines in soybean roots and soil[J]. Plant Pathology, 2003,52: 74–83. DOI: 10.1046/j.1365-3059.2003.00797.x |




