文章信息
- 周世文, 李瑞宁, 刘晓英, 焦学磊, 徐志刚
- ZHOU Shiwen, LI Ruining, LIU Xiaoying, JIAO Xuelei, XU Zhigang
- 光谱能量分布对大豆胚尖再生体系的影响
- Effects of spectral energy distribution on soybean embryonic tip regeneration system
- 南京农业大学学报, 2017, 40(1): 27-33
- Journal of Nanjing Agricultural University (Social Science), 2017, 40(1): 27-33.
- http://dx.doi.org/10.7685/jnau.201604013
-
文章历史
- 收稿日期: 2016-04-05
大豆[Glycine max(L.)Merr.]再生体系是大豆遗传转化的基础,如何建立一个快速高效的大豆再生体系为大豆的遗传改良提供优良的平台已成为研究热点。但现有的大豆组培再生体系还普遍存在再生率低、重复性差、基因型依赖性强、培养条件复杂和再生周期长等问题,很大程度上限制了利用基因工程手段对大豆的遗传改良[1-2]。大豆胚尖再生体系是近几年发展起来的新技术体系[3],具有出芽快、伸长容易、生长周期短和重复性好等优点,受到越来越多的人关注,但胚尖再生体系出芽数略少[4-5]。
前人研究了基因型、培养基组分、激素种类和浓度等对大豆再生体系的影响[5-8],而光对大豆再生体系影响的研究却少有报道。光不仅为组培植物的光合作用提供能量,还作为环境信号调控植物的生命活动,对植物生长过程中的物质代谢、形态建成和基因表达等都有着重要的影响[8-16]。Smith[8]研究认为红光能促进茎和细胞的伸长,而蓝光具有相反的效果。Muleo等[11]的研究表明红光有利于植物茎节间伸长,蓝光虽然抑制了植物茎节间伸长但却有利于茎节的发育。并且许多研究表明复合LED光处理更有利于植物的生长发育和物质代谢[12-16]。Matsuda等[14]的研究表明,在红蓝LED复合光下生长的水稻,其叶片光合速率高于单一红光处理。张欢等[15]在对菊花试管苗的研究中发现,红蓝黄复合光有利于丛生苗分化和增殖,对生根组培苗色素形成、生长发育及根系活力也有促进作用。而Kim等[16-17]研究发现,红蓝绿复合光促进了叶用莴苣的生长,并认为绿光可以透过植物冠层照射底部叶片,通过增加底部叶片的光合速率以增加植物生长的潜能。
本文以大豆成熟种子的胚尖作为外植体,采用LED精量调制光谱分布,探究了不同光谱能量分布对大豆胚尖再生体系的影响,以期筛选出适宜的光谱能量分布,为大豆遗传转化体系的优化奠定基础。
1 材料与方法 1.1 材料试验采用大豆品种为‘南农99-10号’,种子由南京农业大学作物遗传与种质创新国家重点实验室提供。
1.2 试验设计挑选籽粒完好、大小一致的大豆种子,在37 ℃的烘箱中保存8~12 h后进行灭菌。采用氯气灭菌法:将选取的大豆种子放在培养皿中,量取100 mL NaClO放于广口瓶中,与放好种子的培养皿平稳地放在通风橱内密闭的容器中,量取10 mL浓盐酸混于盛有次氯酸钠的广口瓶中,迅速密封容器,灭菌时间为8~10 h。将灭菌好的大豆放入添加了3.0 mg · L-1 6-BA和0.5 mg · L-1IBA的无菌水中,于无菌培养室中放置24 h。在无菌工作台上,将浸泡24 h后吸水膨胀的大豆去掉种皮,去除子叶和原叶,分离得到胚尖外植体,将胚尖外植体转入胚尖诱导培养基(MS+B5+0.5 mg · L-1 6-BA+0.2 mg · L-1 IBA+30 g · L-1蔗糖+8 g · L-1琼脂,pH5.8)中。每瓶诱导培养基放置6个胚尖外植体,置于不同光谱分布处理下培养21 d。
在诱导21 d后,将诱导出芽的胚尖外植体切除底部老化组织,转入伸长培养基中,伸长培养基采用不添加任何激素的MS+B5培养基。待丛生芽长至3~5 cm,将丛生芽转移至生根壮苗培养基(MS+B5+0.5 mg · L-1 IBA+30 g · L-1蔗糖+8 g · L-1琼脂,pH5.8),并置于不同光谱分布处理下培养21 d。
试验所用的各种波长LED光源由南京欧谱润生物科技有限公司研制并提供,调制后的各试验处理组光谱处理的参数如表 1所示,以普通荧光灯作为对照。调节电压、电流、占空比、光源与植物之间的距离,将各种光源的总光密度统一设置为50 μmol · m-2 · s-1,光/暗时间为12 h/12 h。培养室的相对湿度为(75±5)%,温度为(25±2)℃。
| 光谱处理 Light treatment | 光密度/(μmol·m-2·s-1)Photosynthetic photon flux density(PPFD) | 总光密度/(μmol·m-2·s-1)Total PPFD | ||||
| R660 | R630 | B | Y | G | ||
| R660B | 40 | 10 | 50 | |||
| R630B | 40 | 10 | 50 | |||
| R660BY | 33.4 | 8.3 | 8.3 | 50 | ||
| R630BY | 33.4 | 8.3 | 8.3 | 50 | ||
| R660BG | 33.4 | 8.3 | 8.3 | 50 | ||
| R630BG | 33.4 | 8.3 | 8.3 | 50 | ||
| W | 50 | |||||
| 注: R660:660 nm红光 Red light;R630:630 nm红光 Red light;B:蓝光 Blue light;Y:黄光 Yellow light;G:绿光 Green light;W:白光White light | ||||||
每个处理组随机选取培养21 d的胚尖外植体,记录芽再生率、平均芽数、芽长度。
芽再生率=出芽外植体数/接种外植体数×100%,
平均芽数=总芽数/诱导出芽的外植体总数(总芽数为诱导出的芽(长度≥3 mm)的总数)。
每个处理组随机选取10株生根苗进行形态指标测定和生物量分析,包括株高、茎粗、主根数、主根长、主根直径,样品在85 ℃的烘箱中烘干至恒质量,用电子天平(Shimadzu AUY120)称量。测量时各处理样本均随机采样,每个处理重复3次。
根系活力测定采用四氮唑法(TTC)[18],叶绿素含量采用无水乙醇丙酮提取法[18]测定。
每个处理随机取3株植株,在相同部位选取3片健康完整的叶片观察气孔。叶片的上、下表皮用酒精棉擦拭干净,涂上透明指甲油,风干后用透明胶带按压到叶片上,制成临时切片[19],采用Olympus DP71显微镜在30个视野中统计单位叶面积内的气孔数目,由Motic Images Plus 2.0(Olympus Inc.,Japan)软件统计分析数据。气孔频数用单位叶面积内的气孔数计算(mm-2)。
1.4 数据整理与分析采用Excel 2007和SPSS 20.0软件进行数据整理与分析,然后进行方差分析(ANOVA),采用Duncan′s法分析差异显著性。
2 结果与分析 2.1 光谱能量分布对大豆胚尖丛生芽诱导的影响从表 2可见:各处理的大豆胚尖丛生芽再生率为62.50%~79.17%,处理间无显著差异。单株平均芽数以R660BY处理最佳(4.05),其次是R660BG处理(3.58)。R660BY和R660BG处理的平均芽数分别高于R630BY和R630BG处理,并且在含有660 nm红光的光谱处理中,R660BY处理的平均芽数显著高于R660BG及R660B处理;R660BG处理的平均芽数高于R660B处理。表明在添加黄光或绿光的情况下,660 nm红光比630 nm红光更有利于胚尖丛生芽芽数的诱导,在红蓝光的基础上添加黄光比绿光更有利于芽数的增加。
| 光处理 Light treatment | 芽再生率/% Induction rate of shoots | 单株平均芽数 Average shoots per plant |
| W | 62.50±6.67a | 3.29±0.61bc |
| R660B | 70.83±13.33a | 3.37±0.69bc |
| R630B | 62.50±10.67a | 2.75±0.52c |
| R660BY | 75.00±12.25a | 4.05±0.89a |
| R630BY | 75.00±15.63a | 3.33±0.75bc |
| R660BG | 79.17±17.67a | 3.58±0.91b |
| R630BG | 62.50±10.50a | 3.25±0.60bc |
| 注:同列不同字母表示处理间在0.05水平差异显著。 | ||
| Note:Different letters in the same column indicate statistically significant difference at 0.05 level. The same as follows. | ||
R660BY和R660BG处理的总芽数最高,R630B处理最低(图 1),丛生芽的总芽数表现出与丛生芽平均芽数相近的规律。
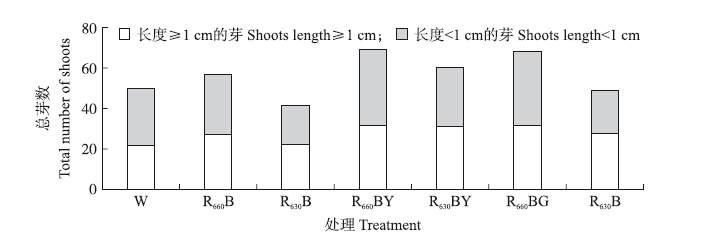
|
图 1 光谱能量分布对大豆胚尖丛生芽数的影响 Figure 1 Effect of the spectral energy distribution on the number of the multiple shoot clumps from embryonic tips of soybean |
含有660 nm红光的处理组中,芽长>1 cm的芽数均多于含有630 nm红光的相应处理组,表明660 nm红光更有利于丛生芽的伸长。
2.2 光谱能量分布对大豆胚尖再生苗生长的影响大豆再生苗的单株主根数以R660B处理最佳(5.21),高于R660BY和R660BG,但主根的长度表现出相反结果,R660B最差(8.48 cm),其次是W和R630B处理(表 3)。表明在红蓝光的基础上添加黄光或绿光不利于主根数的增加,但有利于主根的伸长。并且R630BY和R630BG的主根长度显著大于R660BY和R660BG,表明在添加黄、绿光的情况下,630 nm红光更有利于根系的伸长。主根直径、干质量和根系活力均以R660BY处理最佳,R660BY和R630BY处理大于R660BG和R630BG处理,表明在红蓝光的基础上添加黄光更有利于主根的直径、干质量和根系活力的增加。并且R660B、R660BY和R660BG处理的干质量和根系活力分别高于R630B、R630BY和R630BG处理,表明660 nm红光更有利于干质量的增加,促进根系活力。
| 光处理 Light treatment | 株高/cm Plant height | 茎粗/mm Stem diameter | 主根数 Number of main roots | 主根长/cm Length of main roots | 主根直径/mm Main root diameter | 干质量/mg Dry mass | 根系活力/ (g·g-1·h-1) Root activity |
| W | 6.29±0.94c | 1.14±0.07ab | 2.93±0.44c | 9.47±0.86cd | 0.75±0.05bc | 65.63±1.71c | 1.45±0.17bc |
| R660B | 9.07±0.47ab | 1.08±0.07b | 5.21±0.63a | 8.48±0.79d | 0.70±0.04bc | 75.32±6.11abc | 2.11±0.24ab |
| R630B | 7.32±1.00bc | 1.13±0.04ab | 4.17±0.49abc | 10.13±0.76cd | 0.61±0.06c | 68.30±4.30bc | 1.48±0.07bc |
| R660BY | 9.32±0.50ab | 1.21±0.04ab | 4.64±0.58ab | 11.33±1.16c | 0.99±0.06a | 84.29±5.04a | 2.34±0.09a |
| R630BY | 8.82±0.86ab | 1.28±0.05a | 4.01±0.45abc | 13.04±0.30ab | 0.96±0.11a | 71.73±2.92abc | 1.85±0.15ab |
| R660BG | 10.09±0.29a | 1.11±0.03ab | 4.54±0.63ab | 11.06±0.67bc | 0.88±0.07ab | 82.22±4.37ab | 1.96±0.24ab |
| R630BG | 8.22±0.43abc | 1.19±0.09ab | 3.36±0.39bc | 14.05±0.55a | 0.83±0.09abc | 69.65±1.93bc | 1.25±0.12c |
R630BG处理的叶绿素a、叶绿素b和叶绿素总量最高(图 2),与W处理间差异不显著,但显著高于R630BY以及其他处理组。表明在复合光谱中,与660 nm红光相比,630 nm红光更有利于叶绿素含量的积累。
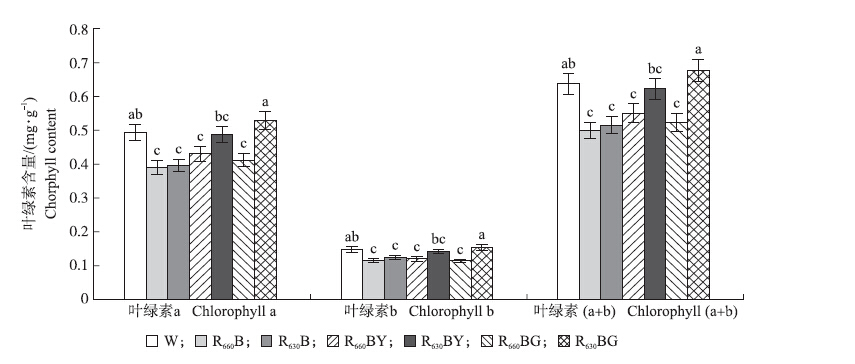
|
图 2 光谱能量分布对大豆胚尖再生苗叶绿素含量的影响 Figure 2 Effect of the spectral energy distribution on the chlorophyll content of soybean regenerated plants |
R660B、R660BY和R660BG处理的气孔宽和面积分别大于R630B、R630BY和R630BG处理,并且R660BY和R630BY处理的气孔长、宽和面积高于R660BG和R630BG处理(表 4),表明660 nm红光和黄光更有利于生根苗气孔的发育。
各处理的气孔频数均高于W对照,并且R660BG和R630BG处理的气孔频数高于R660BY和R630BY处理。表明复合光谱能促进气孔频数的增加,在红蓝光的基础上添加绿光能显著促进气孔频数的增加(P<0.05)。
| 光处理 Light treatment | 气孔长/μm Length of a stoma | 气孔宽/μm Width of a stoma | 气孔长/宽 Length to width ratio | 气孔面积/μm2 Area of a stoma | 气孔频数/mm-2 Stomata of frequency |
| W | 11.83±0.34bc | 7.41±0.34cd | 1.63±0.09ab | 71.19±3.83bcd | 282.52±11.26d |
| R660B | 12.46±0.34b | 8.72±0.56abc | 1.43±0.08abc | 77.22±3.27b | 342.07±18.08bc |
| R630B | 11.21±0.58c | 8.51±0.34bc | 1.32±0.09c | 70.31±5.45bcd | 360.99±30.17abc |
| R660BY | 14.89±0.67a | 9.99±0.54a | 1.48±0.08abc | 86.08±7.40a | 354.36±32.78abc |
| R630BY | 12.39±0.56b | 8.34±0.39bc | 1.50±0.08abc | 76.41±4.85bc | 366.60±20.45abc |
| R660BG | 11.83±0.70bc | 8.82±0.44ab | 1.36±0.11bc | 68.87±2.75bcd | 407.63±13.38a |
| R630BG | 11.61±0.69bc | 6.94±0.33d | 1.65±0.12a | 57.91±5.69d | 420.42±9.63a |
丛生芽的诱导是大豆再生体系的基础,直接影响着大豆再生体系的再生率及转化率。Burritt等[20]研究表明,不同的光谱分布不仅影响芽原基形成的数目,也影响芽原基的扩展。芽是直接由表皮细胞形成,而细胞应对器官性的刺激是直接暴露在光下,不是在外植体或愈伤组织的内部[21],从而导致光对芽的形成产生影响。试验中我们发现,不同复合光谱处理对大豆胚尖的芽再生率并无显著差异。660 nm红光比630 nm红光更有利于胚尖丛生芽芽数的诱导和芽的伸长,并且在红蓝光的基础上添加黄光有利于丛生芽的诱导。问涛等[22]研究表明复合光谱中630 nm的红光抑制大豆子叶节丛生芽之间相互伸长,而660 nm的红光能缓解这一效应,并且促进丛生芽的生长。徐志刚等[23]研究发现黄光有利于文心兰原球茎分化出芽,本研究结果与之相一致。
大豆再生体系中的生根阶段对提高组培苗的再生效率和移栽驯化成活率具有重要作用。有研究表明光谱能够显著影响植物根系生长[24-25]。并且红光能促进植物离体培养的增殖和生根阶段的株高和节间的伸长[17,26-28]。问涛等[22]研究表明,复合光谱中660 nm红光更有利于大豆子叶节再生苗形态生长、干物质积累和根系发育。我们的试验发现660 nm红光更有利于胚尖再生苗的干物质积累和根系发育,630 nm红光能促进根系的伸长,660 nm红光更有利于大豆胚尖再生苗的生长。这可能是因为660 nm红光是植物对光吸收的一个峰值,更有利于植物的生长发育。试验中我们还发现,在红蓝光的基础上添加黄光更有利于主根直径、干质量和根系活力的增加,这与在红蓝复合光的基础上添加黄光不仅有利于丛生苗的分化和增殖,也利于促进生根苗的生长发育和根系活力的研究结果[15]相一致。
大豆苗中叶绿素的含量和气孔的发育影响其光合能力、健康程度和移栽成活率。前人对大豆生根苗叶片的研究较少。刘晓英等[29]和张欢等[15]研究表明,在红蓝光的基础上添加其他光质有利于植株光合色素的积累,促进光合作用。然而我们在试验中发现复合光谱不利于叶绿素含量的积累,与660 nm红光相比,630 nm红光更有利于叶绿素含量的积累。这可能是因为640~660 nm红光是植物叶绿素对光波的一个最强吸收区域,630 nm红光相比于660 nm红光,叶片的利用率较低。在本研究中,复合光谱能促进气孔频数的增加,660 nm的红光有利于再生苗气孔的发育,并且在红蓝光的基础上添加黄光更有利于气孔的发育,添加绿光则显著促进气孔频数的增加。常涛涛等[30]研究表明,红蓝绿复合光下番茄幼苗叶片气孔密度较高,气孔面积较大。这说明除了红蓝光外,黄光和绿光也影响着植物气孔的发育。虽然含有660 nm红光的处理组叶绿素含量较低,但气孔发育良好,促进了光合作用,并且有利于植物与外界环境的交流,这也许是660 nm红光更有利于大豆胚尖再生苗生长的原因。
| [1] | Paz M M, Shou H, Guo Z, et al. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant[J]. Euphytica, 2004,136(2): 167–179. DOI: 10.1023/B:EUPH.0000030670.36730.a4 |
| [2] | 练云, 梁慧珍, 王树峰, 等. 农杆菌介导大豆遗传转化研究进展及转基因作物现状[J]. 大豆科学, 2009, 28(3): 531–536. Lian Y, Liang H Z, Wang S F, et al. Advances on Agrobacterium-mediated genetic transformation of soybean and present status of genetically modified crops[J]. Soybean Science, 2009,28(3): 531–536. (in Chinese) |
| [3] | 刘海坤, 卫志明. 利用根癌农杆菌介导转化大豆成熟种子胚尖获得转基因植株[J]. 植物生理与分子生物学报, 2004, 30(6): 631–636. Liu H K, Wei Z M. Transgenic soybean obtained with Agrobacterium tumefaciens-mediated transformation of embryonic tip of soybean mature seeds[J]. Journal of Plant Physiology and Molecular Biology, 2004,30(6): 631–636. (in Chinese) |
| [4] | 马晓红, 姚陆铭, 武天龙. 大豆整个子叶节外植体再生体系的建立及与子叶节、胚尖再生体系的比较[J]. 大豆科学, 2008, 27(3): 373–378, 379. Ma X H, Yao L M, Wu T L. High frequency plant regeneration from whole cotyledonary node explants and comparison with cotyledonary node and embryonic tip regeneration system in soybean[Glycine max (L.) Merrill][J]. Soybean Science, 2008,27(3): 373–378, 379. (in Chinese) |
| [5] | 闫帆, 孙昕, 翟莹, 等. 大豆胚尖再生体系的研究[J]. 大豆科学, 2011, 30(5): 757–759. Yan F, Sun X, Zhai Y, et al. Optimization on the regeneration system of soybean embryonic tips[J]. Soybean Science, 2011,30(5): 757–759. (in Chinese) |
| [6] | 邱波, 王志坤, 孟凡立, 等. 不同大豆基因型再生性及对农杆菌敏感性的研究[J]. 大豆科学, 2011, 30(5): 752–756. Qiu B, Wang Z K, Meng F L, et al. Regeneration and sensitivity to Agrobacterium of different soyean genotypes[J]. Soybean Science, 2011,30(5): 752–756. (in Chinese) |
| [7] | 聂王星, 於丙军. TDZ和6-BA对大豆子叶节再生体系中丛生芽诱导的效应[J]. 南京农业大学学报, 2012, 35(4): 130–134. Nie W X, Yu B J. Effects of TDZ and 6-BA on inducing multiple shoots in soybean cotyledonary node regeneration system[J]. Journal of Nanjing Agricultural University, 2012,35(4): 130–134. DOI: 10.7685/j.issn.1000-2030.2012.04.024 (in Chinese) |
| [8] | Smith H. Light quality, photoperception, and plan strategy[J]. Annual Review of Plant Physiology, 2003,33(1): 481–518. |
| [9] | Chory J, Wu D. Weaving the complex web of signal transduction[J]. Plant Physiology, 2001,125: 77–80. DOI: 10.1104/pp.125.1.77 |
| [10] | 刘晓英, 焦学磊, 徐志刚, 等. 不同红蓝LED光照强度对樱桃番茄幼苗生长和抗氧化酶活性的影响[J]. 南京农业大学学报, 2015, 38(5): 772–779. Liu X Y, Jiao X L, Xu Z G, et al. Effects of different red and blue LED light intensity on growth and antioxidant enzyme activity of cherry tomato seedings[J]. Journal of Nanjing Agricultural University, 2015,38(5): 772–779. DOI: 10.7685/j.issn.1000-2030.2015.05.011 (in Chinese) |
| [11] | Muleo R, Morini S. Physiological dissection of blue and red light regulation of apical dominance and branching in M9 apple rootstock growing in vitro[J]. Journal of Plant Physiology, 2008,165(17): 1838–1846. DOI: 10.1016/j.jplph.2008.01.007 |
| [12] | 刘晓英, 吴丹, 焦学磊, 等. 不同光谱能量分布对水稻秧苗生长的影响[J]. 南京农业大学学报, 2015, 38(5): 735–741. Liu X Y, Wu D, Jiao X L, et al. Effect of different spectral energy distribution on growth and development of rice seeding[J]. Journal of Nanjing Agricultural University, 2015,38(5): 735–741. DOI: 10.7685/j.issn.1000-2030.2015.05.006 (in Chinese) |
| [13] | 徐文栋, 刘晓英, 焦学磊, 等. 蓝光量对莴苣生长和品质的影响[J]. 南京农业大学学报, 2015, 38(6): 890–895. Xu W D, Liu X Y, Jiao X L, et al. Effect of blue light quality on growth and quality of lettuce[J]. Journal of Nanjing Agricultural University, 2015,38(6): 890–895. DOI: 10.7685/j.issn.1000-2030.2015.06.003 (in Chinese) |
| [14] | Matsuda R, Ohashi-Kaneko K, Fujiwara K, et al. Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light[J]. Plant and Cell Physiology, 2005,45(12): 1870–1874. |
| [15] | 张欢, 徐志刚, 崔瑾, 等. 不同光谱能量分布对菊花试管苗增殖及生根的影响[J]. 园艺学报, 2010, 37(10): 1629–1636. Zhang H, Xu Z G, Cui J, et al. Effects of light spectral energy distribution on multiplication and rooting of chrysanthemum plantlets in vitro[J]. Acta Horticulturae Sinica, 2010,37(10): 1629–1636. (in Chinese) |
| [16] | Kim H H, Goins G D, Wheeler R M J C. Stomatal conductance of lettuce grown under or exposed to different light qualities[J]. Annals of Botany, 2004,94(5): 691–697. DOI: 10.1093/aob/mch192 |
| [17] | Kim H H, Goins G D, Wheeler R M, et al. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes[J]. HortScience, 2004,39(7): 1617–1622. |
| [18] | Ma X, Wang Y, Liu M, et al. Effects of green and red lights on the growth and morphogenesis of potato (Solanum tuberosum L.) plantlets in vitro[J]. Scientia Horticulturae, 2015,190: 104–109. DOI: 10.1016/j.scienta.2015.01.006 |
| [19] | Liu X Y, Guo S R, Xu Z G, et al. Regulation of chloroplast ultrastructure, cross-section anatomy of leaves, and morphology of stomata of cherry tomato by different light irradiations of light-emitting diodes[J]. HortScience, 2011,46: 217–221. |
| [20] | Burritt D J, Leung D W M. Adventitious shoot regeneration from Begonia×erythrophylla petiole sections is developmentally sensitive to light quality[J]. Physiologia Plantarum, 2003,118: 289–296. DOI: 10.1034/j.1399-3054.2003.00083.x |
| [21] | Burritt D J, Leung D W M. Organogenesis in cultured petiole explants of Begonia×erythrophylla:the timing and specificity of the inductive stimuli[J]. Journal of Experimental Botany, 1996,47: 557–567. DOI: 10.1093/jxb/47.4.557 |
| [22] | 问涛, 焦学磊, 刘晓英, 等. 光谱分布对大豆子叶节再生的影响[J]. 大豆科学, 2015, 34(5): 826–832. Wen T, Jiao X L, Liu X Y, et al. Effects of light spectra on soybean cotyledonary node regeneration system[J]. Soybean Science, 2015,34(5): 826–832. (in Chinese) |
| [23] | 徐志刚, 崔瑾, 邸秀茹. 不同光谱能量分布对文心兰组织培养的影响[J]. 北京林业大学学报, 2009, 31(4): 45–50. Xu Z G, Cui J, Di X R. Effects of different spectral energy distribution on tissue culture of Oncidium in vitro[J]. Journal of Beijing Forestry University, 2009,31(4): 45–50. (in Chinese) |
| [24] | Liu X Y, Guo S R, Chang T T, et al. Regulation of the growth and photosynthesis of cherry tomato seedlings by different light irradiations of light emitting diodes (LED)[J]. African Journal of Biotechnology, 2012,22(11): 6169–6177. |
| [25] | Johkan M, Shoji K, Goto F, et al. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa[J]. Environmental and Experimental Botany, 2012,75: 128–133. DOI: 10.1016/j.envexpbot.2011.08.010 |
| [26] | Kuril Ač ik A, Miklušyt-AČG2 anova R, Žilevskait S, et al. In vitro cultivation of grape culture under solid-state lighting[J]. SodininkystIr Daržininkyste, 2007,26(3): 235–245. |
| [27] | Kim S J, Hahn E J, Heo J W, et al. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro[J]. Scientia Horticulturae, 2004,101: 143–151. DOI: 10.1016/j.scienta.2003.10.003 |
| [28] | Silva M H M D, Debergh P C. The effect of light quality on the morphogenesis of in vitro cultures of Azorina vidalii (Wats.) Feer[J]. Plant Cell, Tissue and Organ Culture, 1997,51(3): 187–193. DOI: 10.1023/A:1005988621036 |
| [29] | 刘晓英, 徐志刚, 常涛涛, 等. 不同光质LED弱光对樱桃番茄植株形态和光合性能的影响[J]. 西北植物学报, 2010, 30(4): 725–732. Liu X Y, Xu Z G, Chang T T, et al. Growth and photosynthesis of cherry tomato seedling exposed to different low light of LED light quality[J]. Acta Botanica Boreali-Occidentalia Sinica, 2010,30(4): 725–732. (in Chinese) |
| [30] | 常涛涛, 刘晓英, 徐志刚, 等. 不同光谱能量分布对番茄幼苗生长发育的影响[J]. 中国农业科学, 2010, 43(8): 1748–1756. Chang T T, Liu X Y, Xu Z G, et al. Effects of light spectral energy distribution on growth and development of tomato seedlings[J]. Scientia Agricultura Sinica, 2010,43(8): 1748–1756. (in Chinese) |




